Draw a heating curve (such as the one in Figure 12.36) for 1 mol of benzene beginning
Question:
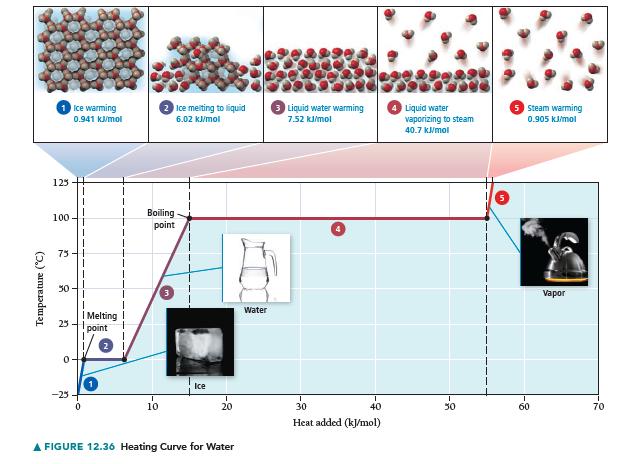
Draw a heating curve (such as the one in Figure 12.36) for 1 mol of benzene beginning at 0 °C and ending at 100 °C. Assume that the values given here are constant over the relevant temperature ranges.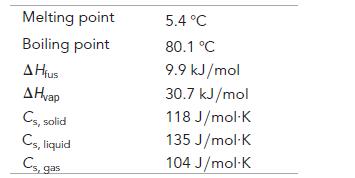
Transcribed Image Text:
Melting point Boiling point A Hius A Hvap Cs, solid Cs, liquid Cs, gas 5.4 °C 80.1 °C 9.9 kJ/mol 30.7 kJ/mol 118 J/mol K 135 J/mol K 104 J/mol-K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Temp Heat added 0 solid 0 54 solid 118 Jmol 1 K 1 x 1mol x 54 0C 6372 J 54 L...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a heating curve (such as the one in Figure 12.36) for 1 mole of methanol beginning at 170 K and ending at 350 K. Assume that the values given here are constant over the relevant temperature...
-
Based on Westlaw's data on the case Christoff v. Nestl USA, INC., I did the brief case. My question is, is there any need to add and adjust the content and form of the case brief, especially the...
-
Of what use, if any, are models such as the one in Figure 1-3 to managers?
-
What is the benefit to a company from a securities underwriter? They study the market and advise companies on where to set their IPO share price. They help companies to reduce the risk associated...
-
Four separate cases involving similar fact situations were consolidated as they presented the same constitutional question. In each case, police officers, detectives, or prosecuting attorneys took a...
-
Consider the use of CF2Cl2 as a dispersing agent for aerosol spray cans. Estimate the pressure a can has to withhold at 40C. Its enthalpy of vaporization at its normal boiling point (244 K) is State...
-
What is the difference between manifest and latent content?
-
When Harriet went away for the summer, Landry, a house painter, painted her house. He had a contract to paint a neighbors house but painted Harriets house by mistake. When Harriet returned from...
-
The company's adjusted trial balance includes the following account balances: Cash, $15,000; Equipment, $85,000: Accumulated Depreciation, $25,000: Accounts Payable, $10,000; Retained earnings....
-
1. Create and upload a histogram of the salary data for the city of Bell, where each bar width is about 50,000 US dollars. (Data for the histogram is at the bottom). a.) Is the distribution of the...
-
Air conditioners not only cool air but dry it as well. A room in a home measures 6.0 m * 10.0 m * 2.2 m. If the outdoor temperature is 30 C and the partial pressure of water in the air is 85% of the...
-
A sample of steam with a mass of 0.552 g and at a temperature of 100 C condenses into an insulated container holding 4.25 g of water at 5.0 C. Assuming that no heat is lost to the surroundings, what...
-
Find the critical points and apply the Second Derivative Test (or state that it fails). (x) = sin x cos 3 x, [0, ]
-
Hogan Business Systems has a small number of sales on account but is mostly a cash business. Consequently, it uses the direct write-off method to account for uncollectible accounts. During 2011 Hogan...
-
Bourret Inc. experienced the following events for the first two years of its operations. 2011: 1. Provided \(\$ 60,000\) of services on account. 2. Provided \(\$ 25,000\) of services and received...
-
A flowrate of lubricant oil is \(5 \mathrm{~kg} / \mathrm{s}\) passing through a Venturi tube. The ratio of areas of the Venturi tube is \(0.005 \mathrm{~m}^{2} /0.002 \mathrm{~m}^{2}\). The density...
-
Sketch on separate axes, in the interval 0 360, the graphs of: In each case show the coordinates of any maximum and minimum points, and of any points at which the curve meets the axes. a y = sec...
-
The wheel of radius \(r=300 \mathrm{~mm}\) rolls to the right without slipping and has a velocity \(v_{O}=3 \mathrm{~m} / \mathrm{s}\) of its center \(O\). Calculate the velocity of point \(A\) on...
-
Give the structural formulas of the alkenes that, on ozonolysis, give: a. (CH3)2C=O and CH2=O b. Only (CH3CH2)2C=O c. CH3CH=O and CH3CH2CH=O d. O=CHCH2CH2CH2CH=O
-
Construct a 4 x 25 design confounded in two blocks of 16 observations each. Outline the analysis of variance for this design.
-
Identify the product(s) that would be formed when each of the following compounds is treated with aqueous acid: (a) Methyl -d-glucopyranoside (b) Ethyl -d-galactopyranoside
-
Consider the structures of the four d-aldopentoses (See the following figure). (a) Which d-aldopentose produces the same aldaric acid as d-lyxose? (b) Which d-aldopentoses yield optically inactive...
-
Trehalose is a naturally occurring disaccharide found in bacteria, insects, and many plants. It protects cells from dry conditions because of its ability to retain water, thereby preventing cellular...
-
A company has $60 billion of sales and $3 billion of net income. It total assets are $ 30 billion. The companys total assets equal total invested capital, and its capital consists of half debt and...
-
Project ABC Initial End-of-Year Investment Cash Flows for years 1-3, respectively $47,000 $20,000 30,000 24,000 WACC = 14% What is the NPV? (Please round to the nearest dollar and do not enter the...
-
A semi-annual coupon bond has 15 years left to maturity. Its coupon rate is 6.5%. If you require an annual rate of return at 7% for your investment. What is this bond's intrinsic value to you? Please...

Study smarter with the SolutionInn App


