Draw the Lewis structure for each organic compound from its condensed structural formula. a. CH4 d. CH3CHOH
Question:
Draw the Lewis structure for each organic compound from its condensed structural formula.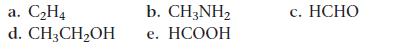
Transcribed Image Text:
a. C₂H4 d. CH3CH₂OH b. CH3NH₂ e. HCOOH c. HCHO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a HCCH O ...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure for each organic compound from its condensed structural formula. a. C3H8 d. CH3COOH b. CH3OCH3 e. CH3CHO C. CH3COCH3
-
Draw the Lewis structure for each of the following molecules or ions, and predict their electron-domain and molecular geometries: (a) AsF3, (b) CH3+, (c) BrF3, (d) ClO3- (e) XeF2, (f) BrO2-.
-
An organic compound is found to contain 37.5 percent carbon, 3.2 percent hydrogen, and 59.3 percent fluorine by mass. The following pressure and volume data were obtained for 1.00 g of this substance...
-
Would you ever expect to find a tumor-inducing retrovirus that carried a processed cellular tumor suppressor gene in its genome?
-
Use the Customers table in the BSN database that accompanies this book and the Report Wizard to create the report in Figure. Note that you will have to reformat and perhaps reposition several labels,...
-
Use Worksheet 5.1. Art Winkler is trying to decide whether to lease or purchase a new car costing $18,000. If he leases, hell have to pay a $600 security deposit and monthly payments of $425 over the...
-
Which of your qualifications do you think are of particular importance for the open position in our company?
-
In 2018, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2020. Information related to the contract is...
-
The B/C ratio for a flood control project along the Swanee River was calculated to be 1.6. If the benefits were $490,000 per year and the maintenance costs were $221,000 per year, determine the...
-
1 (10 points) Brenan, Inc. purchased equipment at the beginning of 2004 for $2,100,000. Brenan. The equipment has an estimated residual value (salvage value) of $100,000 and an estimated life of 5...
-
Use Lewis structures to explain why Br 3 and I 3 are stable, while F 3 is not.
-
The cyanate ion (OCN ) and the fulminate ion (CNO ) share the same three atoms but have vastly different properties. The cyanate ion is stable, while the fulminate ion is unstable and forms...
-
You are asked to develop a multiple regression model that indicates the relationship between a person's behavioral characteristics and the daily cost of food (daily cost). The predictor variables to...
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
Consider the steady-state diffusion of hydrogen through the walls of a cylindrical nickel tube as described in Problem 6.D2. One design calls for a diffusion flux of 5 10-8 mol/m2-s, a tube radius...
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
In SI units, velocity is measured in units of meters per second (m/s). Which of the following combinations of units can also be used to measure velocity? (a) Cm/s (b) Cm/s 2 (c) m 3 /(mm 2 s 2 ) (d)...
-
A typical airplane can fly at a speed of 400 miles per hour. What is its speed in meters per second?
-
Figure P2.13 shows three motion diagrams, where the dots indicate the positions of an object after equal time intervals. Assume left-to-right motion. For each motion diagram, sketch the appropriate...
-
Kaidan Inc. is a Japanese law firm located in Osaka. The firm received JPY 3,000,000 cash for legal services to be rendered in the future. According to the firm's records, the full amount was...
-
What if a contract requires a specific airline for shopping goods, and that airline goes bankrupt before goods were shipped?
-
Based on the npv rule, you should invest in a project with an npv of -1,254. True or false

Study smarter with the SolutionInn App


