Estimate the crystal field splitting energy (in kJ/mol) for a complex ion that is red in solution.
Question:
Estimate the crystal field splitting energy (in kJ/mol) for a complex ion that is red in solution.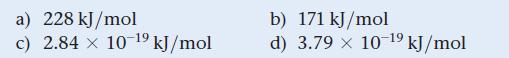
Transcribed Image Text:
a) 228 kJ/mol c) 2.84 x 10-¹9 kJ/mol b) 171 kJ/mol d) 3.79 x 10-¹⁹ kJ/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 16% (6 reviews)
a ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1)How many geometric isomers are there for each species? Part A [Fe(CO)4Cl2] Part B [Pt(NH3)2Cl2Br2]+ 2) Which of the following complex ions absorbs light of the longestwavelength? Which of the...
-
The complex ion [Cu(NH 3 ) 6 ] 2 + is blue in aqueous solution. Estimate the crystal field splitting energy (in kJ/mol) for this ion.
-
Choose a DOW 30 company or you may already have it and predict their return (LN (price today/price yesterday) for today and compare it with the actual return on the stock price. Elaborate and perform...
-
Holly funded the Holly Marx Trust in January 2020. The entire trust income is payable to her adult son Jack for 20 years. At the end of the twentieth year, the trust assets are to pass to Hollys...
-
The reports that follow are from a grocery store. Which report would be used for financial purposes, and which would be used for activities-based decision making?Why? s 1,000 Scan grocery purchases...
-
In 1973, the President of Texaco, Inc., made a statement to a U.S. Senate subcommittee concerned with air and water pollution. The committee was concerned with, among other things, the noise levels...
-
Big heads? The army reports that the distribution of head circumference among male soldiers is approximately Normal with mean 22.8 inches and standard deviation 1.1 inches. Use the 689599.7 rule to...
-
Is Blood Pressure the Same for Both Arms? Listed below are systolic blood pressure measurements (mm Hg) taken from the right and left arms of the same woman (based on data from Consistency of Blood...
-
You are considering buying common stock in Grow On, Inc. You have calculated that the firm's free cash flow was $5.20 million last year. You project that free cash flow will grow at a rate of 18.0%...
-
Explain the differences between each pair of isomer types. a. Structural isomer and stereoisomer b. Linkage isomer and coordination isomer c. Geometric isomer and optical isomer
-
Using the Lewis acidbase definition, how would you categorize a ligand? How would you categorize a transition metal ion?
-
The Conch Oil Company, located at Marathon in the Florida Keys, contracted to sell and to deliver 500 barrels of fuel oil on the first of each month for one year to the Monsoon Mushroom Factory, an...
-
Lennys Limousine Service (LLS) is considering the purchase of two Hummer limousines. Various information about the proposed investment follows: Required: Help LLS evaluate this project by calculating...
-
Lancer Corp. has the following information available about a potential capital investment Required: 1. Calculate the projects net present value. 2. Without making any calculations, determine whether...
-
Woodchuck Corp. is considering the possibility of outsourcing the production of upholstered chair pads included with some of its wooden chairs. The company has received a bid from Padalong Co. to...
-
Woodchuck Corp. is considering eliminating a product from its line of outdoor tables. Two products, the Oak-A and Fiesta tables, have impressive sales. However, sales for the Studio model have been...
-
Suppose that Flyaway Company also produces the Windy model fan, which currently has a net loss of \($40,000\) as follows: Eliminating the Windy product line would eliminate \($20,000\) of direct...
-
Following are the concepts of accounting covered in Chapters 2 through 5. Match each transaction or definition with its related concept by entering the appropriate letter in the space provided. Use...
-
If |62x|>9, which of the following is a possible value of x? A. 2 B. 1 C. 0 D. 4 E. 7
-
Two small particles have charges Q 1 = +3.0 C and Q 2 = -5.0 C. If the magnitude of the electric force between the particles is 120 N, what is the distance between the particles?
-
The particles in Problem 19 are conducting and are brought together so that they touch. Charge then moves between the two particles so as to make the excess charge on the two particles equal. If the...
-
Three charges with q = +7.5 ?C are located as shown in Figure P17.21, with L = 25 cm.? (a) What are the magnitude and direction of the total electric force on the charge at the bottom?? (b) What are...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


