Find the amount (in moles) of CC bonds that must be broken when 1.0 mole of C(g)
Question:
Find the amount (in moles) of C—C bonds that must be broken when 1.0 mole of C(g) is formed from C(diamond). Calculate the ΔH of sublimation of diamond from the data in Appendix II, Table B. Then do the calculation using the C—C bond energy in Table 10.3. Suggest a reason for the difference between the two values.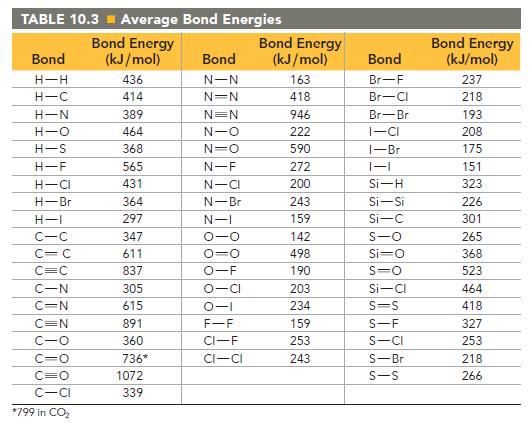
Transcribed Image Text:
TABLE 10.3 Average Bond Energies Bond Energy (kJ/mol) 436 414 Bond H-H H-C H-N H-O H-S H-F H-CI H-Br H-I C-C C C C=C C-N C-N C=N C-O CIO C=O C-CI *799 in CO₂ 389 464 368 565 431 364 297 347 611 837 305 615 891 360 736* 1072 339 Bond N-N N=N N=N N-O N=O N-F N-CI N-Br N-I 0-0 0-0 O-F O-CI 0-1 F-F CI-F CI-CI Bond Energy (kJ/mol) 163 418 946 222 590 272 200 243 159 142 498 190 203 234 159 253 243 Bond Br-F Br-Cl Br-Br I-CI 1-Br 1-1 Si-H Si-Si Si-C S-O Si-O S=O Si-Cl S-S S-F S-CI S-Br S-S Bond Energy (kJ/mol) 237 218 193 208 175 151 323 226 301 265 368 523 464 418 327 253 218 266
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
20 mol of CC bonds 715 kJmol 69 10 2 kJ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
McGuire Industries prepares budgets to help manage the company. McGuire is budgeting for the fiscal year ended January 31, 2021. During the preceding year ended January 31, 2020, sales totaled $9,200...
-
Refer to the statement of stockholders equity for Crisanti Corporation in Exhibit 14-4 to answer the following questions: (1) At what price per share were the 10,000 shares of common stock sold? (2)...
-
For the population in Example 2.2, find the sampling distribution of for a. an SRS of size 3 (without replacement) b. an SRSWR of size 3 (with replacement). For each, draw the histogram of the...
-
The type of process loss that should not affect cost of inventories is (a) Standard loss (b) Seasonal loss (c) Normal loss (d) Abnormal loss
-
The Great Lakes Company, a grocery store chain, purchases apples from a produce distributor in Virginia. The grocery company has an agreement with the distributor that it desires shipments of 10,000...
-
lu Sep. 1 Received $41,000 cash and issued common stock to Stanley Sep. 4 Purchased office supplies, $1,000, and furniture, $1,600, on account. Sep. 6 Performed services for a law firm and received...
-
Using the molecular orbital model for a diatomic molecule, explain the different bond lengths for the ions of oxygen. Also state which ion is diamagnetic. lon O+ 0 0 0- -O Bond Length (pm) 112 121...
-
Sodium peroxide is a very powerful oxidizing agent. Balance the reaction of sodium peroxide with elemental iron to give sodium oxide and Fe 3 O 4 .
-
To foster ethical behavior among employees, managers should apply ethical standards to which they are committed.(True/False)
-
Which of the five hazardous attitudes do you display most frequently? What can you do to minimize the presence and impact of these attitudes in your life?
-
What brought you to this course? How do you define Black or Blackness? What do you hope to get out of this class? When you think of Black Culture, what is the first thing that comes to mind? [For...
-
1. What is XBRL Taxonomy? How do you as a preparer of financial statement use the XBRL Taxonomy in locating a label for a specific financial element? 2. What are the benefits of adopting XBRL from...
-
What a business can do to protect and minimize the invasion of privacy for their customers? Think of your experience when visiting a website. What do most websites have you agree to before you do...
-
Based on your interest, skill set, or goals what do you typically contribute when working in groups? What do you need others to contribute due to your lack of interest, skill set, or goals? How do...
-
Pratt Computer Sales, Inc., reported the following financial statements for 2016: Compute the following investing cash flows; enter all amounts in thousands. a. Acquisitions of plant assets (all were...
-
You have just begun your summer internship at Omni Instruments. The company supplies sterilized surgical instruments for physicians. To expand sales, Omni is considering paying a commission to its...
-
A ball of positive charge rotates about a vertical axis (Fig. P20.61). (a) If the rotation is clockwise as viewed from above, what is the direction of the balls magnetic moment? (b) If the charge on...
-
. Velocity selector. Consider a charged particle moving through a region in which the electric field is perpendicular to the magnetic field, with both fields perpendicular to the initial velocity of...
-
You wish to design a velocity selector (see Problem 62 and Fig. P20.62) that will allow protons to pass through only if they have a speed of 500 m/s. (a) If the magnetic field is B = 0.050 T, what...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


