Find the density (in g/cm 3 ) of a metal cylinder with a mass (m) of 8.3
Question:
Find the density (in g/cm3) of a metal cylinder with a mass (m) of 8.3 g, a length (l ) of 1.94 cm, and a radius (r) of 0.55 cm. For a cylinder, V = πr2l.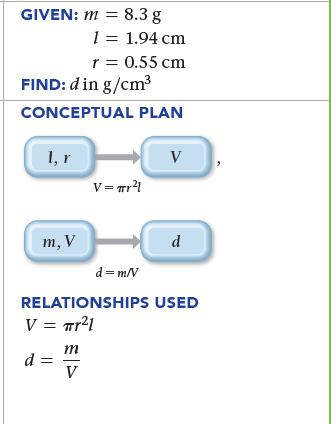
Transcribed Image Text:
GIVEN: m = 8.3 g 1, r FIND: d in g/cm³ CONCEPTUAL PLAN m, V 1 = 1.94 cm r = 0.55 cm m V V = Tr²1 V d d = m/V RELATIONSHIPS USED V = Tr²1 d
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
SOLUTION V wr21 T055 cm 194 cm 18436 cm d ...View the full answer

Answered By

Diksha Bhasin
I have been taking online teaching classes from past 5 years, i.e.2013-2019 for students from classes 1st-10th. I also take online and home tuitions for classes 11th and 12th for subjects – Business Studies and Economics from past 3 years, i.e. from 2016-2019. I am eligible for tutoring Commerce graduates and post graduates. I am a responsible for staying in contact with my students and maintaining a high passing rate.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A sample consisting of 22.7 g of a nongaseous, unstable compound X is placed inside a metal cylinder with a radius of 8.00 cm, and a piston is carefully placed on the surface of the compound so that,...
-
An aluminum cylinder (SG = 2.7) slides concentrically down a taut 1-mmdiameter wire as shown in the figure. Its length is L = 8 cm and its radius R = 1 cm. A 2-mm-diameter hole down the cylinder...
-
Figure P17.73 shows a cylindrical capacitor; it consists of a solid metal rod of radius r 1 surrounded by a metal cylinder with inner radius r 2 and outer radius r 3 . Suppose the capacitor has...
-
Evaluate and simplify the following derivatives. d (4u + u du 8u+ 1/
-
Corvette Collection of Boston, Inc. (CCB), was a used-Corvette dealership located (despite its name) in Pompano Beach, Florida. In addition to selling used Corvettes, CCB serviced Corvettes and sold...
-
What nominal annual rate of interest was charged on a loan of $5600 repaid in end- of-month installments of $121.85 in four-and- a-half years?
-
List the advantages and disadvantages of centralising organisational functions.
-
Everett Co. was organized on July 1, 2014. Quarterly financial statements are prepared. The unadjusted and adjusted trial balances as of September 30 are shown below. Instructions (a) Journalize the...
-
Prior to liquidation, the partners' capital balances are reported as follows: ARIANA - Capital, P210,000, P&L Ratio, 1/4; GRANDE - Capital, P240,000, P&L Ratio, 3/4. The total liabilities of the...
-
Ann Riat - Salary Comparison The director of the company has asked you to confirm with Ann if she prefers to keep her annual salary of $64,000 and agrees to receive no commission on future...
-
The first diagram depicts a compound in its liquid state. Which of the other diagrams best depicts the compound after it has evaporated into a gas? (a) (b)
-
Explain the difference between an element and a compound.
-
What are the five components of the driverless car IS, and which lessons from Chapter 2 can be applied to the concept of a Google car?
-
Consider a hydraulic car jack with a piston area ratio of 50. A person can lift a 1000-kg car by applying a force of (a) \(100 \mathrm{kgf}\) (b) \(10 \mathrm{kgf}\) (c) \(50 \mathrm{kgf}\) (d) \(20...
-
to the original three-level scale and compare the results from the two versions of the depression diagnosis variable.
-
Under the race set up in Section8.1.2, think a scenario when you may have left truncation issue. 8.1.2 Truncation Another issue arising in the analysis of time to event data is truncation. Under...
-
Use mean score, IPW, and MI methods to estimate the sensitivity and specificity of the test in Example 11.1. Example 11.1 Suppose that we are interested in estimating the prevalence of a disease...
-
Assess the test-retest reliability for each of the domains of CSF-36 based on the study described in Example10.5. Example 10.5 Consider the random subsample of 197 patients in the CSF-36 study who...
-
Winning times for men and women in the 1500 m Olympic speed skating event are given below, in minutes and seconds. a. Analyze the data and predict when the winning times for men and women will be the...
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
Consider the reaction: 2 H 2 S (g) 2 H 2 (g) + S 2 (g) Kc = 1.67 10 -7 A reaction mixture initially contains [H 2 S] = 0.010 M. Find the equilibrium concentrations of H 2 and S 2 .
-
Calculate the pH of each solution and indicate whether the solution is acidic or basic at 25 C. a) [H 3 O + ] = 0.012 M b) [H 3 O + ] = 3.7 10 -7 M c) [OH - ] = 0.0046 M d) [OH - ] = 8.1 10 -8 M
-
Find the [H 3 O + ] and pH of a 1.00 M formic acid solution.
-
Plant Co completed a special landscaping job for Smith Co. Plant uses ABC and has the following activity rates: Activity Allocation Base Activity Rate Designing Number of designs $275 / design...
-
HELP ASAP On January 15, the end of the first biweekly pay period of the year, North Company's payroll register showed that its employees earned $50,000 of sales salaries Withholdings from the...
-
These account balances listed below were provided to you from the Horizon Corporation at the end of December 31, 2020. Salaries and wages payable$ 2,580 Salaries and wages expense39,850 Utilities...

Study smarter with the SolutionInn App


