The first diagram depicts a compound in its liquid state. Which of the other diagrams best depicts
Question:
The first diagram depicts a compound in its liquid state.
Which of the other diagrams best depicts the compound after it has evaporated into a gas?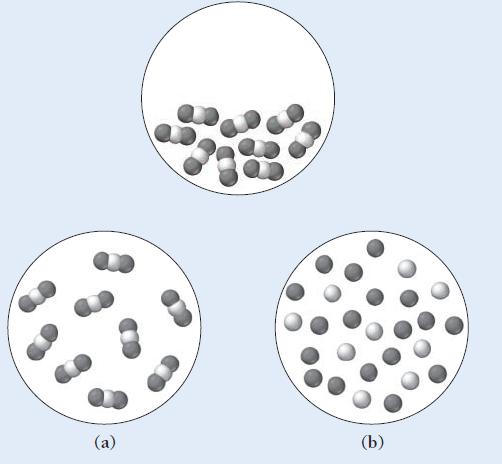
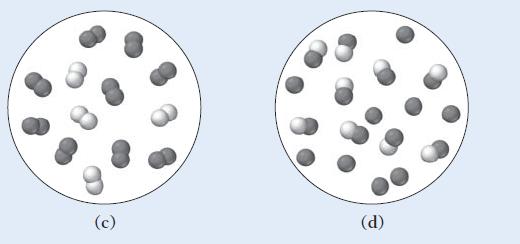
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Task 3 Critically evaluate ethical decision - making at Apple and recommend how current ethical challenges can be effectively managed.
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Use implicit differentiation to find dy/dx. 6x 3 + 7y 3 = 13xy
-
William Bisby gave an all-terrain vehicle (ATV) to Del City Cycle in Enid, Oklahoma, to sell on his behalf. Joseph Maddox bought the ATV but paid for it with a check written on a closed checking...
-
Compute the nominal annual rate of interest compounded monthly at which $400 paid at the end of the month for eight years accumulates to $45 000.
-
Several forms of coordination are described. Select two that you have seen in operation and describe how they work and how well they work.
-
1. Emerson Process Management, a global supplier of measurement, analytical, and monitoring instruments and services based in Austin, Texas, had a new data warehouse designed for analyzing customer...
-
Bridgeport Company issued $ 672,000 of 10%, 20-year bonds on January 1, 2020, at 102. Interest is payable semiannually on July 1 and January 1. Bridgeport Company uses the effective-interest method...
-
Lakey International is a foreign corporation that maintains its books of record in foreign currency (FC), although its functional currency is the U.S. dollar. Lakey originally began operations on...
-
Find the radius (r) in centimeters of a spherical water droplet with a volume (V ) of 0.058 cm 3 . For a sphere, V = (4>3) r 3 . GIVEN: V = 0.058 cm FIND: r in cm CONCEPTUAL PLAN V 4 v=mr V= 3 r...
-
Find the density (in g/cm 3 ) of a metal cylinder with a mass (m) of 8.3 g, a length (l ) of 1.94 cm, and a radius (r) of 0.55 cm. For a cylinder, V = r 2 l. GIVEN: m = 8.3 g 1, r FIND: d in g/cm...
-
What is interest rate parity and what happens when this condition doesnt hold?
-
Show that the Wald statistic in (4.15) does not depend on the specific equations used. Specifically, suppose that \(K\) and \(K^{\prime}\) are two equivalent systems of equations for a linear...
-
Mia Sales experienced the following events during 2011, its first year of operation: 1. Started the business when it acquired \(\$ 50,000\) cash from the issue of common stock. 2. Paid \(\$ 21,000\)...
-
In 2011, Grice Incorporated sold land for \(\$ 95,000\) cash. The land had originally cost \(\$ 50,000\). Also, Grice sold inventory that had cost \(\$ 200,000\) for \(\$ 275,000\) cash. Operating...
-
To obtain the log-linear models for association homogeneity, we need the following two key facts: (a) Prove (7.25). (b) Prove that \(\lambda_{i^{\prime} j k}^{x y z}+\lambda_{i j^{\prime} k}^{x y...
-
For the DOS, use the three-level depression diagnosis and variable MS for marital status as defined in Section 4.2.2 to test for uniform association, assuming that both the depression diagnosis and...
-
Suppose the long-distance phone companies in Example A calculate their charges so that a call of exactly 3 min will cost the same as a call of 3.25 min or 3.9 min, and there is no increase in cost...
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Consider the reaction: CO (g) + 2 H 2 (g) CH 3 OH (g) A reaction mixture initially contains [CO] = 0.600 M and [H 2 ] = 1.20 M. At equilibrium, the CO concentration is found to be 0.100 M. Find the...
-
Consider the reaction: 2 COF 2 (g) CO 2 (g) + CF 4 (g) Kc = 2.00 In an equilibrium mixture, the concentration of COF 2 is 0.35 M and the concentration of CO 2 is 0.144 M. What is the equilibrium...
-
Consider the reaction: N 2 O 4 (g) 2 NO 2 (g) Kc = 0.36 A reaction mixture initially contains [N 2 O 4 ] = 0.100 M. Find the equilibrium concentrations of N 2 O 4 and NO 2 .
-
Need help answering Part B Thank you Multiple Production Department Factory Overhead Rate Method Four Finger Appliance Company manufactures small kitchen appliances. The product line consists of...
-
Described below are six independent and unrelated situations involving accounting changes. Each change occurs during 2021 before any adjusting entries or closing entries were prepared. Assume the tax...
-
Swifty Corporation was organized on January 1, 2021. During its first year, the corporation issued 2,300 shares of $50 par value preferred stock and 140,000 shares of $10 par value common stock. At...

Study smarter with the SolutionInn App


