For each of the reactions, calculate the mass (in grams) of the product that forms when 3.67
Question:
For each of the reactions, calculate the mass (in grams) of the product that forms when 3.67 g of the underlined reactant completely reacts. Assume that there is more than enough of the other reactant.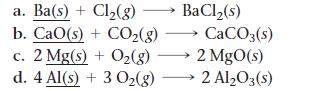
Transcribed Image Text:
a. Ba(s) + Cl₂(g) b. CaO(s) + COz(g) CaCO3(s) c. 2 Mg(s) + O₂(g) →→→ 2 MgO(s) d. 4 Al(s) + 3 O₂(g) 2 Al₂O3(s) BaCl₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 556 g Ba...View the full answer

Answered By

SILPA MARY THOMAS
I have done my graduation and post-graduation in Chemistry. I have one year research experience and 4+ years teaching experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the reactions, calculate the mass (in grams) of the product that forms when 15.39 g of the underlined reactant completely reacts. Assume that there is more than enough of the other...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Hernandez Company began 2010 with a $120,000 balance in retained earnings. During the year, the following events occurred: 1. The company earned net income of $80,000. 2. A material error in net...
-
Romo, Inc., has current assets of $1,850, has current assets of $1,850, net fixed assets of $8,600, current liabilities of $1,600, and long-term debt of $6,100. What is the value of the shareholders...
-
A piston - cylinder device initially contains steam at 200 kPa, 200oC, and 0.4 m3. At this state, a linear spring (F x) is touching the piston but exerts no force on it. Heat is now slowly...
-
Cowboy Exploration, Ltd, plans to replace some aging but still serviceable oil-field equipment now while it still has a salvage value. The companys investment analyst has made the following estimates...
-
We are studying mutual bond funds for the purpose of investing in several funds. For this particular study, we want to focus on the assets of a fund and its five-year performance. The question is:...
-
Which of the following statements is false The price of a security should equal to the present value of its cash flows, up to the transaction costs of trading the security and the cash flows. In most...
-
(a) In the circuit in Fig. 4.71, calculate vo and Io when vs = 1 V. (b) Find vo and io when vs = 10 V. (c) What are vo and Io when each of the 1-Ω resistors is replaced by a...
-
Find the limiting reactant for each initial amount of reactants. a. 2 mol Na, 2 mol Br 2 b. 1.8 mol Na, 1.4 mol Br 2 c. 2.5 mol Na, 1 mol Br 2 d. 12.6 mol Na, 6.9 mol Br 2 2 Na(s) + Br(g) Br(g) 2...
-
Sulfuric acid dissolves aluminum metal according to the reaction: Suppose you want to dissolve an aluminum block with a mass of 15.2 g. What minimum mass of H 2 SO 4 (in g) do you need? What mass of...
-
Find the sum of the series. 3" 5" n! -0
-
2. For the following three sets of electric field lines, what charge or charges would make such lines? Indicate their locations and type of charge (e.g. positive/negative) a.
-
What is the most important take-home point that you learned from this video? https://www.youtube.com/watch?v=nUZqvsF_Wt0 2. Policy Problems. What is onepolicy that creates inequality in the labor...
-
An employee had $20,300 in gross earnings up to September 20, 2021. She has the following information for her pay for the week ending September 27, 2021. Her employer contributes 100% toward CPP and...
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
A cross was made between two strains of plants that are agriculturally important. One strain was disease-resistant but herbicide-sensitive; the other strain was disease-sensitive but...
-
Solve the relation Exz:Solve therelation ne %3D
-
When 2-ethyl-5-chlorotoluene was treated with sodium hydroxide at high temperature, followed by treatment with H 3 O + , three constitutional isomers with molecular formula C 9 H 12 O were obtained....
-
You wish to design an effusion source for Br atoms from Br 2 (g). If the source is to operate at a total pressure of 7.5 Torr, what temperature is required to produce a degree of dissociation of...
-
Calculate G for the isothermal expansion of 2.25 mol of an ideal gas at 325 K from an initial pressure of 12.0 bar to a final pressure of 2.5 bar.
-
Sam Anderson, your best friend, is considering investing in Advantage Specialties Inc. Sam seeks your advice in interpreting this information. Specifically, she wants to know the company's total...
-
briefly explain five potential Balance sheet/ Income statement items that may have been impacted by COVID 19.
-
At formation of partnership AB. B contributes land ($30,000 FMV and $10,000 basis) and A contributes cash of $30,000. When the land is sold 2 years later for $42,000, A must recognize gain of gow...

Study smarter with the SolutionInn App


