Gallium has two naturally occurring isotopes with the following masses and natural abundances: Sketch the mass spectrum
Question:
Gallium has two naturally occurring isotopes with the following masses and natural abundances: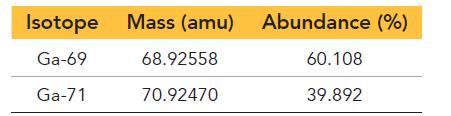
Sketch the mass spectrum of gallium.
Transcribed Image Text:
Isotope Ga-69 Ga-71 Mass (amu) Abundance (%) 60.108 39.892 68.92558 70.92470
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Fig Mass spec...View the full answer

Answered By

Daniel Kimutai
I am a competent academic expert who delivers excellent writing content from various subjects that pertain to academics. It includes Electronics engineering, History, Economics, Government, Management, IT, Religion, English, Psychology, Sociology, among others. By using Grammarly and Turnitin tools, I make sure that the writing content is original and delivered in time. For seven years, I have worked as a freelance writer, and many scholars have achieved their career dreams through my assistance.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Patients seeking care at the County General emergency room wait, on average, 6 minutes before seeing the triage nurse who spends, on average, 4 minutes assessing the severity of their problem. The...
-
Magnesium has three naturally occurring isotopes with the following masses and natural abundances: Sketch the mass spectrum of magnesium. Isotope Mg-24 Mg-25 Mg-26 Mass (amu) 23.9850 24.9858 25.9826...
-
An element has two naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 49.9472. 2.500 103 50.9440. 0.9975 What is the atomic mass of this...
-
Draw structures for the following molecules (a) Acrylonitrile, C3H3N, which contains a carbon-carbon double bond and a carbon-nitrogen triple bond (b) Ethyl methyl ether, C3H8O, which contains an...
-
Rainbow Tours gives walking tours of Springfield. Rainbow charges $40 per person for the tour and incurs $16 in variable costs for labor, drinks, and maps. The monthly fixed costs for Rainbow Tours...
-
When the boss fired Clarence from his job at a moving company, she said it was because he could no longer lift heavy furniture; his salary was too high and, as he got older, he would have a hard time...
-
In what sense does MM's theory represent a middle-ground position between the other two theories? AppendixLO1
-
The following is a list of the items to be included in the preparation of Warrick Company's 2016 statement of cash flow's: a. Net income, $59,200 b. Payment for purchase of building, $98,000 c....
-
or The following information applies to the questions displayed below) 1428 onts Now that operations for outdoor clinics and TEAM events are running smoothly, Suzie thinks of another area for...
-
Code Churn is a common metric used to measure the efficiency and productivity of software engineers and computer programmers. It?s usually measured as the percentage of a programmer?s code that must...
-
Which pair of elements do you expect to be most similar? Why? a. Nitrogen and oxygen b. Titanium and gallium c. Lithium and sodium d. Germanium and arsenic e. Argon and bromine
-
Which pair of elements do you expect to be most similar? Why? a. N and Ni b. Mo and Sn c. Na and Mg d. Cl and F e. Si and P
-
The NASA Team algorithm for calculating sea-ice concentration from SSM/I data defines the polarisation ratio PR as and thegradient ratioGR as where, in both of these expressions,T fP represents the...
-
A vertical solid cylinder of uniform cross-sectional area A floats in water. The cylinder is partially submerged. When the cylinder floats at rest, a mark is aligned with the water surface. The...
-
Non-manufacturing fixed cost for year 2011 equal to:$60,780 out of which half are Administrative expenses.Administrative expenses are expected to increase by: 10%The total Variable nonmanufacturing...
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
Is a random mutation more likely to be beneficial or harmful? Explain your answer.
-
If a process has a six-sigma capability, what is the process capability index? a. 1 b. 2 c. 6 d. 12
-
There are two different compounds with molecular formula C 2 H 6 O. One of these isomers has a much higher boiling point than the other. Explain why.
-
Determine the relationship between the two structures below. Are they resonance structures or are they constitutional isomers?
-
In each reaction, identify the Lewis acid and the Lewis base: (a) (b) (c) F L-
-
Here's how the answer suppose to look like. Fill in the "item" tab. Compromisine Review Problem P O 110 Geronimo Tire Manufacturing Company The comprehensive problem that follows covers the entire...
-
A partnership begins its first year of operations with the following capital balances: Allegan, Capital $ 1 1 0 , 0 0 0 Berrien, Capital 8 0 , 0 0 0 Kent, Capital 1 1 0 , 0 0 0 According to the...
-
a.) Assume that Campbell generated 3% of its total revenue from Mexico in 2021 totaling $245.28 million when translated back to USD. Total revenue in Mexico is expected to increase by 3.25% from 2021...

Study smarter with the SolutionInn App


