Given that the H f of 1 M H 2 SO 3 is -633 kJ, use the
Question:
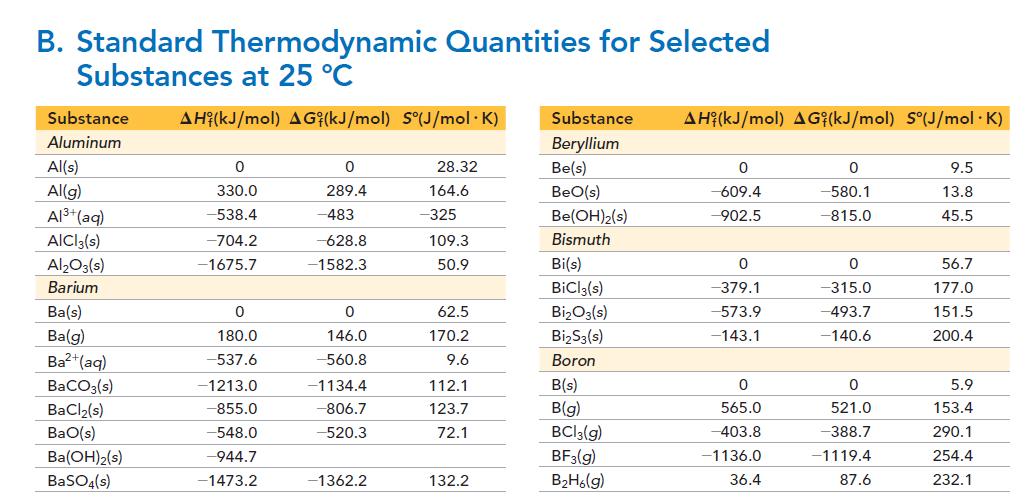
Given that the ΔH°f of 1 M H2SO3 is -633 kJ, use the data in Appendix II, Table B to calculate the ΔH° for the formation of a 1 M solution of SO2 in water from SO2(g).
Transcribed Image Text:
B. Standard Thermodynamic Quantities for Selected Substances at 25 °C Substance AH (kJ/mol) AG (kJ/mol) S°(J/mol.K) Aluminum Al(s) Al(g) Al³+ (aq) AICI 3(s) Al₂O3(s) Barium Ba(s) Ba(g) Ba²+ (aq) BaCO3(s) BaCl₂(s) BaO(s) Ba(OH)2(s) BaSO4(s) 0 330.0 -538.4 -704.2 -1675.7 0 180.0 -537.6 -1213.0 -855.0 -548.0 -944.7 -1473.2 0 289.4 -483 -628.8 -1582.3 0 146.0 -560.8 -1134.4 -806.7 -520.3 -1362.2 28.32 164.6 -325 109.3 50.9 62.5 170.2 9.6 112.1 123.7 72.1 132.2 Substance AH (kJ/mol) AGi(kJ/mol) S°(J/mol .K) Beryllium Be(s) BeO(s) Be(OH)2(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Bi₂S3(s) Boron B(s) B(g) BC13(g) BF3(g) B₂H6(g) 0 -609.4 -902.5 0 -379.1 -573.9 -143.1 0 565.0 -403.8 -1136.0 36.4 0 -580.1 -815.0 0 -315.0 -493.7 -140.6 0 521.0 -388.7 -1119.4 87.6 9.5 13.8 45.5 56.7 177.0 151.5 200.4 5.9 153.4 290.1 254.4 232.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
Use the data in Appendix II, Table B to calculate H for the formation of a 1 M solution of H 2 SO 4 from SO 3 (g). B. Standard Thermodynamic Quantities for Selected Substances at 25 C Substance AH...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In Exercises find the second derivative of the function. f(x) = 8 (x - 2)
-
Develop brief answers to each of the following questions: 1. In what way is selling an investment for a gain potentially a negative in evaluating quality of earnings? 2. Is it unethical for new...
-
Do you think customers might be upset if they found out Lenovo was using remarketing and keeping track of their behavior online? Why or why not?
-
The cost of abnormal process loss is not included in the cost of a process.
-
In a study entitled How Undergraduate Students Use Credit Cards, it was reported that undergraduate students have a mean credit card balance of $3,173 (Sallie Mae, April 2009). This figure was an...
-
2) Financing (5 points) The family has successfully gotten through some tough financial times, but will need to consider some financing in order to continue growing. Describe and make recommendations...
-
Calculate the standard enthalpy of reaction for reducing the different forms of iron oxide to iron metal and CO 2 from the reaction of the oxide with CO. Identify which reaction is the most...
-
Breathing air that contains 0.13% CO by volume for 30 minutes will cause death. CO can form by incomplete combustion of carbon-containing compounds. Calculate the minimum volume of octane (C 8 H 18 ,...
-
(a) Explain why the motion of the engine piston in Figure P15.8 is only approximately simple harmonic motion. (b) What must you do to the length of the connecting rod if you want to make the motion...
-
Perhaps we need a way to differentiate ourselves from the competition? Is it possible that we are dividing the customer's time too much? Does this mean that we should instead look to attract more...
-
Complete these answers with full paragraph sentences. 1)What are the Mission, Vision, & Values of the Palo Alto Network? 2) What are the Four Functions of Management Planning, Organizing, Leading, &...
-
One highly visible trait of a successful leader is that of role model: behavior exhibited by a leader is carefully observed and often sets the tone for the entire center. As a role model, it is...
-
Design a flowchart that illustrates the key processes and decision points within the custom leadership system, along with the various inputs and outputs. At the center of the flowchart is the leader,...
-
Prepare a sample memo to those that have been selected to serve on the "Bulletin 1" committee. Remind them of their charge and outline a calendar of meetings. Lastly, include a list of resources. ...
-
Verifine Corporation reported the following amounts on its 2016 comparative income statements: Perform a horizontal analysis of revenues and net income-both in dollar amounts and in percentages-for...
-
Anna, a high school counselor, devised a program that integrates classroom learning with vocational training to help adolescents at risk for school dropouts stay in school and transition to work...
-
A long, straight wire carries current I = 100 A in a region where the magnetic field has a magnitude B = 10 T, but it is found that the force on the wire is zero. Explain how that can be.
-
A long, straight wire of length 0.75 m carries current I = 1.5 A in a region where B = 2.3 T. If the force on the wire is 1.4 N, what is the angle between the field and the wire?
-
Repeat Problem 47, but assume the force is 5.6 N. Is this possible? Data from Problem 47 A long, straight wire of length 0.75 m carries current I = 1.5 A in a region where B = 2.3 T. If the force on...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


