Have each group member write a problem involving the transfer of heat from one material in Table
Question:
Have each group member write a problem involving the transfer of heat from one material in Table 7.4 to another material in the table. Working as a group, solve each problem. The group member who wrote each problem may act as the group facilitator when the group is working on his or her problem. What do all of your problems have in common? How do they differ?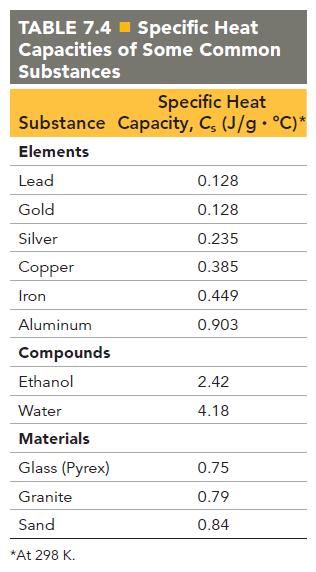
Transcribed Image Text:
TABLE 7.4 Specific Heat Capacities of Some Common Substances Specific Heat Substance Capacity, C, (J/g °C)* Elements Lead Gold Silver Copper Iron Aluminum Compounds Ethanol Water Materials Glass (Pyrex) Granite Sand *At 298 K. 0.128 0.128 0.235 0.385 0.449 0.903 2.42 4.18 0.75 0.79 0.84
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Group Member 1 Problem A copper block 100 g at 100 C is placed in contact with a glass block 200 g a...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
If a particular glucose fermentation process is 87.0% efficient, how many grams of glucose would be required for the production of 51.0 g of ethyl alcohol (C 2 H 5 OH)? C 6 H 12 O 6 2C 2 H 5 OH +...
-
Garza Corporation uses straight-line depreciation for financial reporting purposes but an accelerated method for tax purposes. Is it acceptable to use different methods for the two purposes? What is...
-
If the ratio of net income to sales for a restaurant is 5 percent, the predicted ratio of cash flow from operations (CFO) to sales is closest to: A. 4.054. B. 0.524. C. 4.207. Kenneth McCoin, CFA, is...
-
Arrow Space, Inc., manufactures specialized ceramic components used in the aerospace industry. The companys materials and parts manager is currently revising the inventory policy for XL-20, one of...
-
You have been asked to develop a work breakdown structure for a project. How should you go about accomplishing this? Should the WBS be time-phased, department-phased, division-phased, or some...
-
Define the various terms associated with exchange rates, including spot rates, forward rates, premiums, and discounts.
-
Jack Tasker opened his Auto Repair Shop in November 2023. The balance sheet at November 30, 2023, prepared by an inexperienced part-time bookkeeper, is shown below. Required Prepare a correct balance...
-
In an exothermic reaction, the reactants lose energy, and the reaction feels hot to the touch. Explain why the reaction feels hot even though the reactants are losing energy. Where does the energy...
-
When 1 mol of a gas burns at constant pressure, it produces 2418 J of heat and does 5 J of work. Determine E, H, q, and w for the process.
-
In terms of tort liability, what level of responsibility do auditors owe clients under common law?
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
A company will be financing its operations with and a capital budget is P40,000,000 and a debt-to-equity ratio of 1. The interest rate on company's debt is 10%. The expected return on equity by the...
-
State and explain each rule for determining out-of-control points.
-
Would you use the adjacency matrix structure or the adjacency list structure in each of the following cases? Justify your choice. a. The graph has 10,000 vertices and 20,000 edges, and it is...
-
S p hybridization on each Ge atom in planar trans-digermane has been described as sp 1.5 for the GEGe sigma bond and sp 1.8 for the GeH bond. Calculate the HGeGe bond angle based on this...
-
The energy of the occupied valence MOs of H 2 S is shown as a function of the HSH bond angle. Compared to the analogous diagram, Figure 24.11, for H 2 O, the 2a1 MO energy decreases more as the bond...
-
The following diagram shows the energies of valence molecular orbitals of boron trifluoride. The energies of three occupied orbitals (the a 2 HOMO and doubly degenerate e orbitals) are shown. The...
-
A stock is currently priced at $92.83 per share. The stock paid its annual dividend of $6.32 per share last week. Dividends are expected to grow at a constant rate of 5.20 percent per year in...
-
You have just been appointed the regulatory czar for the financial services Industry. Wave your magic wand and it will be done! What regulations and structures will you add or eliminate? What risks...
-
Happy Lucky Bank has a $1 million position in a five-year, zero-coupon bond with a face value of $1,402,552. The bond is trading at a yield to maturity of 6.00 percent. The historical mean change in...

Study smarter with the SolutionInn App


