How many chiral carbon atoms are in each of the structures in Problem 39? Problem 39 Classify
Question:
How many chiral carbon atoms are in each of the structures in Problem 39?
Problem 39
Classify each saccharide as an aldose or a ketose. Also classify each as a triose, tetrose, pentose, and so on.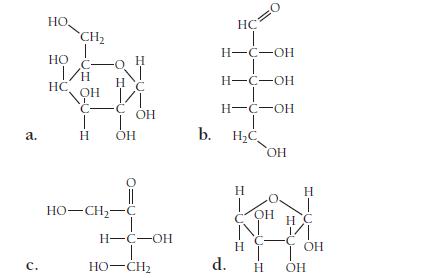
Transcribed Image Text:
a. с. HO. HO CH₂ I/H OH С- T H HC -o H NI HC т оно HO–CH,−C OH H-C-OH I HO-CH2 b. HC H-C-OH H-C-OH H-C-OH T H₂C OH d. Н H OH С T н Н T H C C OH OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 5...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many chiral carbon atoms are in each of the structures in Problem 40? Problem 40 Classify each saccharide as an aldose or a ketose. Also classify each as a triose, tetrose, pentose, and so on. a....
-
Classify each saccharide as an aldose or a ketose. Also classify each as a triose, tetrose, pentose, and so on. a. || CH I H-C-OH HO-C-H H-C-OH HO-C-H CH OH CH | H-C-OH c. HO-CH2 b. d. HO , CH C H OH...
-
Classify each saccharide as an aldose or a ketose. Also classify each as a triose, tetrose, pentose, and so on. a. . HO. HO CH I/H OH - T H HC -o H NI HC HOCH,C OH H-C-OH I HO-CH2 b. HC H-C-OH...
-
Find the derivative of the function. y = e x-4
-
Dodge Company, a small retail bookstore, has experienced losses of inventory over the past year. George Dodge, the owner, on the advice of his accountant, has adopted a set of internal controls in an...
-
Create a vector (name it vtA) that has 10 elements of which the first is 8, the increment is 7, and the last element is 71. Then, assign elements of vtA to a new vector (call it vtB) such that vtB...
-
Under what circumstances is it ethical to use consumer information in marketing research?
-
Brian and Nui Soon live in Sudbury. Two years ago, they visited Thailand. Nui, a professional chef, was impressed with the cooking methods and the spices used in the Thai food. Sudbury does not have...
-
Question 3 1 pts Which of the following accounts may appear on a post-closing trial balance? Cash, Accounts Receivable, and Prepaid Rent Cash, Accounts Receivable, and Rent Revenue Cash, Service...
-
Draw structures for the straight-chain and ring forms of glucose.
-
Draw structures showing the reaction of glycerol with myristic acid to form the triglyceride trimyristin. Would you expect this triglyceride to be a fat or an oil?
-
A blink of an eye is a time interval of about 150 ms for an average adult. The closure portion of the blink takes only about 55 ms. Let us model the closure of the upper eyelid as uniform angular...
-
Simon Company's year-end balance sheets follow. At December 31 Assets Cash Accounts receivable, net Merchandise inventory Prepaid expenses Plant assets, net Total assets Liabilities and Equity...
-
Openthe Phetsimulation Charges and Field from this link: ( https://phet.colorado.edu/en/simulation/charges-and-fields ) . In this simulation, a little different model is used: the little yellow "E...
-
A particle of mass m that is moving along the x-axis is experiencing a restoring force of the form F = -k+x, where kf is the spring constant. The Hamiltonian for this system is given as: = d 2m dx +...
-
Distinguish carefully between the following terms: i) Resolution and Sensitivity. ii) Type A and Type B evaluations of measurement uncertainty. [30%] (b) Describe two common schemes used for the...
-
Write out the form of the partial fraction decomposition of the function (See Example). Do not determine the numerical values of the coefficients. (a) +3 x5 + 2x3 A B C x Dx + E ++++ 2+2 6 (b)...
-
Superior Drive-Ins Ltd. borrowed money by issuing $1,000,000 of 7% bonds payable at 96.5 on July 1, 2016. The bonds are 10-year bonds and pay interest each January 1 and July 1. 1. How much cash did...
-
B made an issue of 150,000 $1 ordinary shares at a premium of 20% the proceeds of which is received by cheque. What is the correct journal to record this? A. Bank Share capital Share premium B. Bank...
-
The human nose is very sensitive to certain molecules. For example, it can sense the presence of the chemical CH 3 SH (methyl mercaptan) at levels as small as 2 parts per billion. Another especially...
-
Bug breath. Insects do not have lungs or a blood circulatory system. Instead, a system of openings in the exoskeleton (spiracles) lead to branching tubes of decreasing diameter called trachea, the...
-
(a) Find the speed for typical Ne, Ar, and Kr atoms and for typical H 2 , LiF, and Cl 2 molecules in the atmosphere at room temperature. (b) Compare these typical speeds to the escape speed for an...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


