How many grams of water form when 1.24 L of H 2 gas at STP completely reacts
Question:
How many grams of water form when 1.24 L of H2 gas at STP completely reacts with O2?![]()
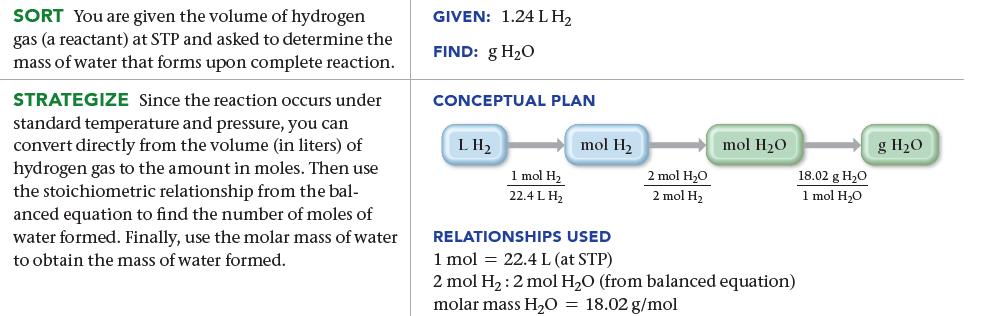
Transcribed Image Text:
2 H₂(g) + O2(8) 2 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
124 LH X 1 mol ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many grams of water form when 2.41 L of oxygen gas at STP completely react with an appropriate amount of H 2 ? 2 H 2 (g) + O 2 (g) 2 H2O(g)
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Lacoste t-shirts come with an average price of $ 120 a piece, at their factory outlet with a std. deviation of $ 17. But at the Seasonal Sale (Discount) outlets of these t- shirts, it was also...
-
Analysts claim that businesses can increase sales on the Internet, but not profits. What evidence does this chapter provide to support or refute this claim? Discuss.
-
Consider the following set of initial user requests from a telephone company about a new customer service system: The telephone company customer service shall be able to record and answer questions...
-
If your friend is successful, causing the name of Porkies to be even better known, thus resulting in greater demand for franchises, should your friend share in future revenue form franchise sales?
-
Comac has decided to enter an extremely competitive market with the C919. What global market strategy do you recommend for Comac as it explores the possibility of marketing its product to major...
-
20. Consider the following information on profit expectations and the probabilities of their occurrence. Profits ($000) 250 250 350 400 600 650 Probabilities 0.10 0.15 0.25 0.25 0.15 0.10 The...
-
The fresh feed to an ammonia production process contain 24.75 mole% N2, 74.25 mole% H2, and the balance inerts (I). The feed is combined with a recycle stream containing the same species, and the...
-
Define molar volume and list its value for a gas at STP.
-
A sample of Xe takes 75 seconds to effuse out of a container. An unknown gas takes 37 seconds to effuse out of the identical container under identical conditions. What is the most likely identity of...
-
What is meant by shareholders funds?
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
Rank the following iron-carbon alloys and associated microstructures from the highest to the lowest tensile strength: (a) 0.25 wt%C with spheroidite, (b) 0.25 wt%C with coarse pearlite, (c) 0.60 wt%C...
-
Cleaning Service Company's Trial Balance on December 31, 2020 is as follows: Account name Debit Credit Cash 700 Supplies Pre-paid insurance Pre-paid office rent Equipment Accumulated depreciation -...
-
Determine the moments at the supports A and C, then draw the moment diagram. Assume joint B is a roller. EI is constant. 25 kN 15 kN/m A. - 4 m- - 3 m -3 m-
-
Determine the moments at A, B, and C, then draw the moment diagram for the beam. The moment of inertia of each span is indicated in the figure. Assume the support at B is a roller and A and C are...
-
Determine the moments at A, B, and C and then draw the moment diagram. EI is constant. Assume the support at B is a roller and A and C are fixed. 3 k 3k B. |- 3 ft--3 ft -3 ft -- -10 ft- 10 ft
-
According to the capital asset pricing model (CAPM), where does an assets expected return come from? Please explain each component.
-
Kappa SA in 2021 had pre-tax profits of 100,000, equity of 450,000 and a return on equity of 20%. How much did equity increase in 2021? Choose one: a. 100,000 b. Not at all c. None of the suggested...
-
Suppose a seven-year, $1,000 bond with a 9.04% coupon rate and semiannual coupons is trading with a yield to maturity of 6.67%. a. Is this bond currently trading at a discount, at par, or at a...

Study smarter with the SolutionInn App


