Question: The fresh feed to an ammonia production process contain 24.75 mole% N2, 74.25 mole% H2, and the balance inerts (I). The feed is combined with
The fresh feed to an ammonia production process contain 24.75 mole% N2, 74.25 mole% H2, and the balance inerts (I). The feed is combined with a recycle stream containing the same species, and the combined stream is fed to a reactor in which a 25% single-pass conversion of N2 is achieved. The products pass through a condenser in which essentially all the ammonia is removed, and the remaining gases are recycled. However, to prevent buildup of the inerts in the system, a purge stream must be taken off. The recycle stream contains 12.5 mole% inerts. Calculate the overall conversion of nitrogen, the ratio (moles purge gas/mole of fresh feed), and the ratio (mole fed to reactor/mole fresh feed).
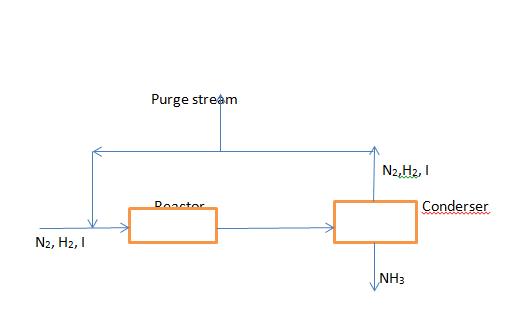
Purge stream N2,H2, I Doactor |Conderser N2, H2, I NH3
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


