Name each compound in which the benzene ring is best treated as a substituent. CH3 | a.
Question:
Name each compound in which the benzene ring is best treated as a substituent.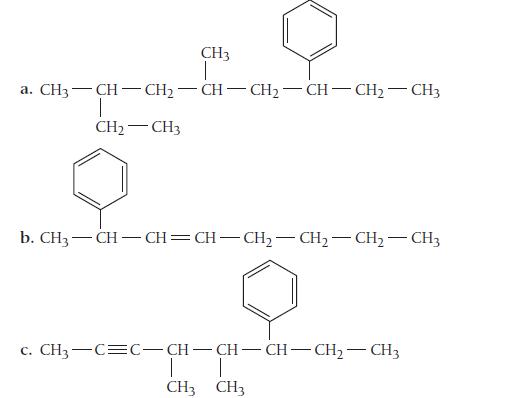
Transcribed Image Text:
CH3 | a. CH3—CH=CH-CH-CH=CH-CH2–CH3 T CH₂CH3 b. CH,—CH—CH=CH–CH,—CH,—CH, CH3 c. CH,—C=C–CH-CH-CH–CH,—CH3 T CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
a 35dimethyl7...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Name each compound in which the benzene ring is best treated as a substituent. a. H3C-CH-CH-CH-CH-CH3 H3C b. CH,CH,CH,CH,C=CCH3 CH3 | c. CH3CH=CH-C=CHCHCH,CH3 T CH3 CH3
-
Give the product of the following reaction: If the terminal sp2 carbon of the substituent bonded to the benzene ring is labeled with 14C, where will the label be in the product? A
-
Arene C has the composition 90.6% carbon and 9.4% hydrogen. Its mass and 1H NMR spectra are shown below. a. b. a. Calculate the empirical formula of C. b. From the mass spectrum, find the molecular...
-
In the year to 5 April 2021, Thomas More made the following disposals: (i) A flat in a house that he had purchased on 1 December 2010 for 80,000. It had never been occupied as the main residence and...
-
Refer to the Kepler Company information as shown in Problem 16-45. Required: 1. Compute the following for each year: a. The times-interest-earned ratio b. The debt ratio 2. Does Kepler have too much...
-
Examine the dimensional model (star schema) shown in Figure 32.13. This model describes part of a database that will provide decision support for a taxi company called FastCabs. This company provides...
-
Firms frequently adapt their products to suit conditions abroad. What other marketing program elements do firms adapt for international markets? LO.1
-
Over its history, Netflix has done a great job of utilizing the elements of the marketing mix to enhance its success. Describe Netflixs marketing mix what product, pricing, distribution, and...
-
Consultex, Incorporated, was founded in 2018 as a small financial consulting business. The company had done reasonably well from 2018 through 2020 but started noticing its cash dwindle early in 2021....
-
Name each disubstituted benzene. a. Br Br b. CH - CH3 CH - CH3 C. F
-
Name each monosubstituted benzene. a. HC-CH3 b. F C. CH3 H3C-C-CH3
-
The bromine-82 nucleus has a half-life of 1.0 10 3 min. If you wanted 1.0 g 82 Br and the delivery time was 3.0 days, what mass of NaBr should you order (assuming all of the Br in the NaBr was 82...
-
Turn this information into an excel sheets with the excel formulas being shown P12.2 (LO 1, 2) (Liability Entries and Adjustments) Listed below are selected transactions of Schultz Department Store...
-
1. Consider an undirected random graph on the set of four vertices {A, B, C, D} such that each of the 4 2 = 6 potential edges exists with probability 0.2, independently of the presence/absence of any...
-
Basic Net Present Value Analysis Jonathan Butler, process engineer, knows that the acceptance of a new process design will depend on its economic feasibility. The new process is designed to improve...
-
Determine the support reactions at the smooth collar A and the normal reaction at the roller support B. 800 N 600 N B 0.8 m 0.4 m 0.4 m 0.8 m
-
A plant hopes to cool a steam line by sending it through a throttling valve to expand it to atmospheric pressure. The steam enters the valve at 550C and 250 bar. The expansion in the valve happens so...
-
After graduation, you face a choice. One option is to work for a multinational consulting firm and earn a starting salary (benefits included) of $40,000. The other option is to use $5,000 in savings...
-
Sportique Boutique reported the following financial data for 2012 and 2011. Instructions(a) Calculate the current ratio for Sportique Boutique for 2012 and 2011.(b) Suppose that at the end of 2012,...
-
A damped LC circuit consists of a 0.15F capacitor and a 20-mH inductor with resistance 1.6 . How many oscillation cycles will occur before the peak capacitor voltage drops to half its initial value?
-
A damped RLC circuit includes a 5.0 resistor and a 100-mH inductor. If half the initial energy is lost after 15 cycles, whats the capacitance?
-
An RLC circuit includes a 1.5-H inductor and a 250F capacitor rated at 400 V. The circuit is connected across a sine-wave generator with V p = 32 V. What minimum resistance will ensure that the...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


