Palladium forms three different compounds with sulfur. The mass of sulfur per gram of palladium in each
Question:
Palladium forms three different compounds with sulfur. The mass of sulfur per gram of palladium in each compound is listed here. Show that these masses are consistent with the law of multiple proportions.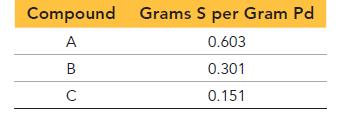
Transcribed Image Text:
Compound Grams S per Gram Pd A 0.603 B 0.301 C 0.151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The law of multiple proportions states that when two elements form a series of compounds the differe...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sulfur and fluorine form several different compounds including sulfur hexafluoride and sulfur tetrafluoride. Decomposition of a sample of sulfur hexafluoride produces 4.45 g of fluorine and 1.25 g of...
-
In a series of experiments, a chemist prepared three different compounds that contain only iodine and fluorine and determined the mass of each element in each compound: (a) Calculate the mass of...
-
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these are decomposed, the sulfur dioxide produces 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produces...
-
Comprehensive variance analysis review. Sonnet Inc. has the following budgeted standards for the month of March 2010: Sales of 2,000,000 units are budgeted for March. Actual March results are: Unit...
-
Austin Enterprises makes and sells three types of dress shirts. Management is trying to determine the most profitable mix. Sales prices, demand, and use of manufacturing inputs follow: The company...
-
Scores on a quiz were normally distributed and had a mean of 10 and a standard deviation of 3. For each of the following scores, find the Z score and the percentage of area above and below the score....
-
Explain this statement: "Using leverage has both good and bad effects. AppendixLO1
-
What are the expected value and variance of the following probability distribution? RANDOM VARIABLE X PROBABILITY 1 ................0.05 2 ................0.05 3 ................0.10 4...
-
A system to reduce the opportunity costs of idle balances through economies of scale derived by aggregating funds into a single account at the bank. Lockbox O Concentration Zero Balance Account O...
-
1. Using the spreadsheet model from Case 2.1 as a starting point, use Solver to find the optimal set of projects to approve. The solution should maximize the total NPV from the approved projects, and...
-
Upon decomposition, one sample of magnesium fluoride produces 1.65 kg of magnesium and 2.57 kg of fluorine. A second sample produces 1.32 kg of magnesium. How much fluorine (in grams) does the second...
-
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produces 28.8 g of sodium upon decomposition. How much fluorine (in grams) forms?
-
It has been estimated that the oil sands in Alberta Canada contain 2 trillion barrels of oil. However, recovering the oil damages the environment. A survey of Canadians and Americans was asked, what...
-
5. [-/0 Points] DETAILS OSPRECALC1 2.2.106. Use algebra to find the point at which the line f(x) = -x 258 -X+ intersects the line h(x) = x+ 91 + 25 10 (x, y) = Additional Materiale MY N
-
What does the graph tells? from your own understanding. CoursHeroTranscribedText 136 DIVIDED ATTENTION COUNTED TIME BACKWARDS 134 1 2 3 130 136 UNDIVIDED ATTENTION COUNTED TIME BACKWARDS 134 5 132...
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
Let's suppose that two strains of pigs differ in 500 RFLPs. One strain is much larger than the other. The pigs are crossed to each other, and the members of the F1 generation are also crossed among...
-
What is the order p of a B + -tree? Describe the structure of both internal and leaf nodes of a B + -tree.
-
Fill in the missing products below. excess HI Heat 1) Hg(OAc), Et 2) NABH, -: Na MCPBA 1) NaSH 2) H20 HBr
-
Propose a plausible synthesis for each transformation. a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. q. r. s. t. u.
-
Propose a structure for ether with molecular formula C 7 H 8 O that exhibits the following 13 C NMR spectrum. Carbon NMR 114.0 129.5, 120,714.0 55.1- 159.71 160 120 140 100 100 Chemical shift (ppm)...
-
On January 1, 2013, Piper Company acquired an 80% interest in Sand Company for $2,291,100. At that time the common stock and retained earnings of Sand Company were $1,801,600 and $731,300,...
-
Ch6) A firms operating income will be Select one: a. greater if higher-contribution-margin units are sold than lower-contribution-margin units. b. equal as long as total sales remain equal,...
-
Skymont Company wants an ending inventory each month equal to 23% of that month's cost of goods sold. Cost of goods sold for February is projected at $90,000. Ending inventory at the end of January...

Study smarter with the SolutionInn App


