Sulfide (S 2- ) salts are notoriously insoluble in aqueous solution. a. Calculate the molar solubility of
Question:
Sulfide (S2-) salts are notoriously insoluble in aqueous solution.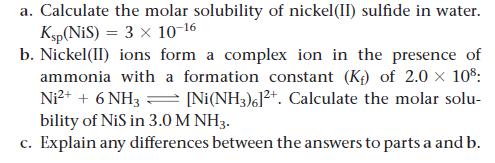
Transcribed Image Text:
a. Calculate the molar solubility of nickel(II) sulfide in water. Ksp(NiS) = 3 x 10-16 b. Nickel(II) ions form a complex ion in the presence of ammonia with a formation constant (K) of 2.0 x 108: Ni²+ + 6 NH3 [Ni(NH3)6]²+. Calculate the molar solu- bility of NiS in 3.0 M NH3. c. Explain any differences between the answers to parts a and b.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a 2 x 10 M b 66 10 M c NiS will dissolve ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
The solubility of a salt in water depends on a broad range of intermolecular bonding forces. These occur between the particles or ions making up the salt, between the salts particles and solvating...
-
Hydrogen sulfide (H 2 S) smells like rotten eggs; its smell can be detected at concentrations as low as 0.02 ppm. Well water, which is drawn from underground depths of 30250 meters (100800 feet), is...
-
Mango Designs began selling its custom furniture on June 1, 2020. At the end of the month, the special journals showed the following results. Other information you will need is as follows: ? Interest...
-
Identify each of the following as (a) An objective of financial statement analysis, (b) A standard for financial statement analysis, (c) A source of information for financial statement analysis, or...
-
A medical research team conducted a study to test the effect of a migraine drug. In the study, 400 subjects took the drug and 407 subjects took a placebo. The results after two hours are shown below....
-
Finally, discuss for which types of products the bookstore would do a straight rebuy and for which it would pursue a modified rebuy.
-
Dr. Lillian Fok, a New Orleans psychologist, specializes in treating patients who are agoraphobic (i.e., afraid to leave their homes). The following table indicates how many patients Dr. Fok has seen...
-
Seved Help Save & Exit Submit Check my work March April, and May have been in partnership for a number of years. The partners allocate all profits and losses on a 4:31 basis, respectively. Recently,...
-
Calculate the solubility of Zn(OH) 2 (s) in 2.0 M NaOH solution. You must take into account the formation of Zn(OH)4, 2 x 105. which has a Kf =
-
Draw a crystal field splitting diagram for a trigonal bipyramidal complex ion. Assume the axial positions are on the z-axis.
-
On June 7, 2013, Cheng and Morales, two recent graduates of Upper State University, formed a computer consulting firm. Cheng contributed an extensive, up-to-date computer installation, valued at...
-
From a square whose side has length \(x\), measured in inches, create a new square whose side is 5 in. longer. Find an expression for the difference between the areas of the two squares as a function...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A circle with radius 5
-
Find the present value of the ordinary annuities in Problems 21-32. Amount of Deposit m 23. $250 Frequency n semiannually Rate r 8% Time t 30 yr
-
Characterize the types of investments that are most vulnerable to political risk. Characterize those that are least vulnerable. What factors influence an investments vulnerability? On a scale of 1 to...
-
Refer to the following tree diagram for a two-stage experiment. Find the probabilities in Problems 1-6. \(P(B) \) E E A B C A B C
-
There are two primary sources of equity reported in the stockholders' equity section of a company's balance sheet: contributed capital and earned capital. Earned capital is kept track of in the...
-
The tractor is used to lift the 150-kg load B with the 24-mlong rope, boom, and pulley system. If the tractor travels to the right at a constant speed of 4 m/s, determine the tension in the rope when...
-
Can an induced electric field exist in the absence of a conductor?
-
A static magnetic field cannot change the energy of a charged particle. Is this true of a changing magnetic field? Discuss.
-
Fluctuations in Earths magnetic field due to changing solar activity can wreak havoc with communications, even those using underground cables. How is this possible?
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


