Calculate the solubility of Zn(OH) 2 (s) in 2.0 M NaOH solution. You must take into account
Question:
Calculate the solubility of Zn(OH)2(s) in 2.0 M NaOH solution.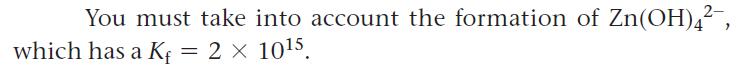
Transcribed Image Text:
You must take into account the formation of Zn(OH)4², 2 x 10¹5. which has a Kf =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To calculate the solubility of ZnOH2s in 20 M NaOH solutionwe must take into account the formation o...View the full answer

Answered By

ABHISHEK GAUTAM
I have an experience for teaching of two years and also i was working on chegg india for last 6 months as SME(subject matter expert) in mechanical engg. subject.I have a great passion for study and teaching .I want to make my career in teaching line itself.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following income statements illustrate different cost structures for two competing companies: Number of customers (a) Sales revenue (a x $230) Variable cost (ax $180) Variable cost (a x $0)...
-
A rigid bar ABCD is being supported by a hinge at A and two cable CE and DE. A measuring gauge determined that the vertical deflection at the free end of the bar is [y] mm. Consider these values: H =...
-
Harvatin Group reported net income totaling $1,000,000 for the year 2006. The following is additional information obtained from the Harvatin Group's financial reports: The Company purchased 100,000...
-
The two roots of a quadratic equation ax 2 + bx + c = 0 can be obtained using the following formula: b 2 - 4ac is called the discriminant of the quadratic equation. If it is positive, the equation...
-
Using 2008 as the base year, prepare a trend analysis of the following data, and tell whether the situation shown by trends is favorable or unfavorable. 2012 20 2010 2009 2008 Net sales Cost of goods...
-
A medical researcher claims that calcium supplements can decrease the systolic blood pressures of men. In part of the study, 10 randomly selected men are given a calcium supplement for 12 weeks. The...
-
What are the major differences between the consumer buying process discussed in Chapter 6 and the B2B buying process discussed in this chapter? Use buying iPads for personal use versus buying more...
-
Assume that a security model is needed for the protection of information in your class. Using the CNSS model, examine each of the cells and write a brief statement on how you would address the three...
-
Your company uses process costing to account for costs of producing products. % Complete You've been given the following information: % Complete Materials Beginning Units in Process 10,000 100%...
-
Explain why [Ni(NH 3 ) 4 ] 2+ is paramagnetic, while [Ni(CN) 4 ] 2- is diamagnetic.
-
Sulfide (S 2- ) salts are notoriously insoluble in aqueous solution. a. Calculate the molar solubility of nickel(II) sulfide in water. Ksp(NiS) = 3 x 10-16 b. Nickel(II) ions form a complex ion in...
-
Are the data consistent with HWE? Specifically, is the number of heterozygotes (TC) significantly lower than expected under HWE? One issue is whether the Y402H gene is in Hardy-Weinberg equilibrium...
-
Use your own academic report, issued by your institute, as an example. Ask a database administrator how they use normalization steps to transform the details of the report into a set of relations in...
-
Francis Corp. has two divisions, Eastern and Western. The following information for the past year is for each division: Francis has established a hurdle rate of 9 percent. Required: 1. Compute each...
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Use values of r cov (Table 17.1) to estimate the XY bond lengths of ClF, BrF, BrCl, ICl and IBr. Compare the answers with values in Fig. 17.8 and Table 17.3, and comment on the validity of the method...
-
From a square whose side has length \(x\), measured in meters, create a new square whose side is \(10 \mathrm{~m}\) longer. Find an expression for the sum of the areas of the two squares as a...
-
During the year, University Food Systems issued stock, repurchased stock, declared a cash dividend, and declared a 2-for-l stock split. How will each of these transactions affect University's...
-
In Exercises discuss the continuity of each function. f(x) -3 1 x - 4 y 3 2 -1 -2 -3+ 3 X
-
In a popular demonstration of induced emf, a lightbulb is connected across a large inductor in an RL circuit, as shown in Fig. 27.37. When the switch is opened, the bulb flashes brightly and may even...
-
You have a fixed length of wire to wind into an inductor. Will you get more inductance if you wind a short coil with large diameter, or a long coil with small diameter?
-
A car battery has a 12-V emf, yet energy from the battery provides the 30,000-V spark that ignites the gasoline. How is this possible?
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


