Table 9.2 does not include francium because none of franciums isotopes are stable. Predict the values of
Question:
Table 9.2 does not include francium because none of francium’s isotopes are stable. Predict the values of the entries for Fr in Table 9.2. Predict the nature of the products of the reaction of Fr with:
(a) Water,
(b) Oxygen, and
(c) Chlorine.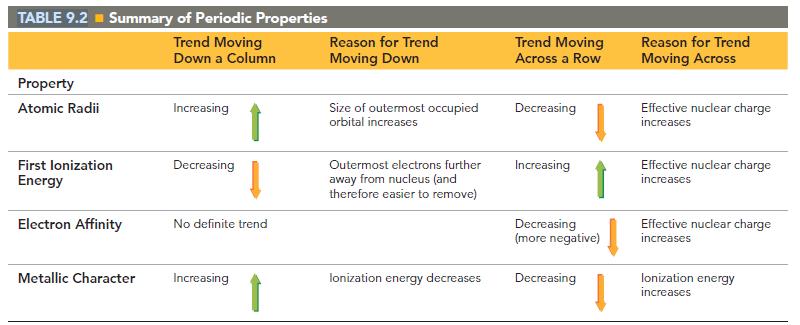
Transcribed Image Text:
TABLE 9.2 = Summary of Periodic Properties Trend Moving Down a Column Property Atomic Radii First lonization Energy Electron Affinity Metallic Character Increasing Decreasing No definite trend Increasing Reason for Trend Moving Down Size of outermost occupied orbital increases Outermost electrons further away from nucleus (and therefore easier to remove) lonization energy decreases Trend Moving Across a Row Decreasing Increasing Decreasing (more negative) Decreasing Reason for Trend Moving Across Effective nuclear charge increases Effective nuclear charge increases Effective nuclear charge increases lonization energy increases
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Fr Rn 7s 26...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
On April 1 of the current taxable year, Mr. Lasing Gho died leaving Php 25, 000, 000 of net distributable estate. He also left behind Tessie, his legitimate wife; Rhealyn, his legally adopted...
-
The R&D division of Nanco Corp. has just developed a chemical for sterilizing the vicious Brazilian killer bees which are invading Mexico and the southern United States. The president of Nanco is...
-
Consider a steady-flow Carnot cycle with water as the working fluid. The maximum and minimum temperatures in the cycle are 350 and 60C. The quality of water is 0.891 at the beginning of the...
-
In your opinion, why have many of the models for knowledge representation come from people with a strong interest in artificial intelligence?
-
On August 1, Jackson Corporation (a U.S.-based importer) placed an order to purchase merchandise from a foreign supplier at a price of 200,000 rupees. Jackson will receive and make payment for the...
-
4. Down Inc. operates a large quarry in Central BC. Selected data from Down Inc. for the year ended December 31, 2018 are presented below: (20 marks) Total assets Average total assets Net earnings...
-
A steel company is producing steel for a new contract. The contract specifies the information in the following table for the steel. The steel company mixes batches of eight different available...
-
The heaviest known alkaline earth metal is radium, atomic number 88. Find the atomic numbers of the as yet undiscovered next two members of the series.
-
We discussed the metalloids, which form a diagonal band separating the metals from the nonmetals. There are other instances in which elements such as lithium and magnesium that are diagonal to each...
-
Students often misunderstand the concept of a one-to-one function (mapping). I think I know the reason. You see, a mapping : A B has a direction associated with it, from A to B. It seems reasonable...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Butlersville Company is considering a capital proposal that has an expected net present value of $3,000. The periodic cash inflows are normally distributed with a standard deviation of $500 each...
-
At Glass Company, materials are added at the beginning of the process and conversion costs are added uniformly. Work in process, beginning: Number of units Transferred - in costs Direct materials...
-
By convention, the anode of a battery is where oxidation takes place. Is this true when the battery is charged, discharged, or both?
-
You wish to maximize the emf of an electrochemical cell. To do so, should the concentrations of the products in the overall reaction be high or low relative to those of the reactants? Explain your...
-
If you double all the coefficients in the overall chemical reaction in an electrochemical cell, the equilibrium constant changes. Does the emf change? Explain your answer.
-
Prepare journal entries to record each of the following transactions of a merchandising company . The company uses a perpetual inventory system and the gross method. Nov. 5 Purchased 1,000 units of...
-
Lululemon, a Canada-based retailer, is facing increased competition from less expensive alternatives to its $100 yoga pants. However, instead of lowering prices or expanding beyond its own 440 stores...
-
Which of the following is calculated by dividing net income by revenues? Multiple Choice Gross profit margin Current ratio Net profit margin Asset turnover

Study smarter with the SolutionInn App


