The osmotic pressure of a solution containing 5.87 mg of an unknown protein per 10.0 mL of
Question:
The osmotic pressure of a solution containing 5.87 mg of an unknown protein per 10.0 mL of solution is 2.45 torr at 25 °C. Find the molar mass of the unknown protein.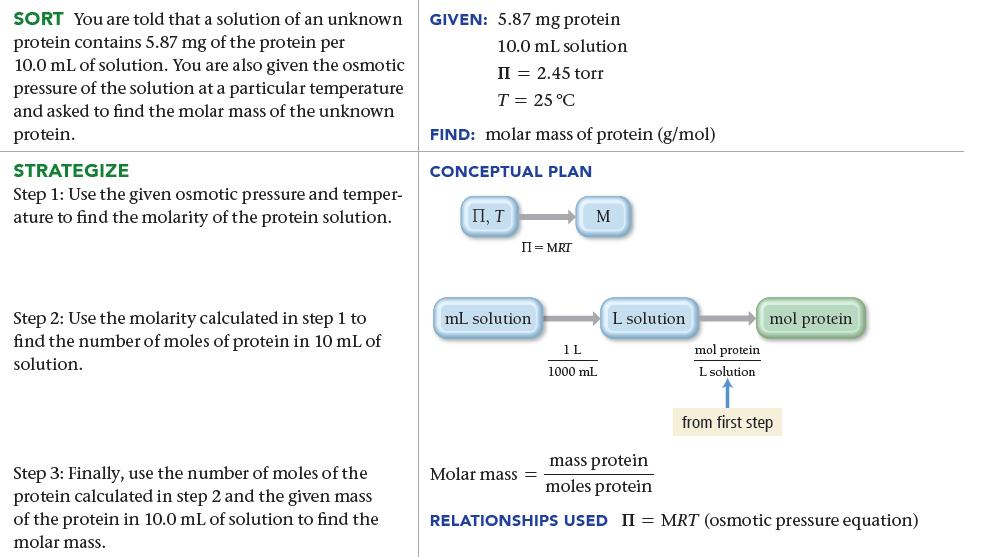
Transcribed Image Text:
SORT You are told that a solution of an unknown protein contains 5.87 mg of the protein per 10.0 mL of solution. You are also given the osmotic pressure of the solution at a particular temperature and asked to find the molar mass of the unknown protein. STRATEGIZE Step 1: Use the given osmotic pressure and temper- ature to find the molarity of the protein solution. Step 2: Use the molarity calculated in step 1 to find the number of moles of protein in 10 mL of solution. Step 3: Finally, use the number of moles of the protein calculated in step 2 and the given mass of the protein in 10.0 mL of solution to find the molar mass. GIVEN: 5.87 mg protein 10.0 mL solution II = 2.45 torr T = 25 °C FIND: molar mass of protein (g/mol) CONCEPTUAL PLAN П, Т II = MRT mL solution. M Molar mass= 1L 1000 mL L solution mol protein L solution mol protein from first step mass protein moles protein RELATIONSHIPS USED II = MRT (osmotic pressure equation)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
II MRT M II RT 245 terf X 100 mL X 008206 ...View the full answer

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The osmotic pressure of a solution containing 22.7 mg of an unknown protein in 50.0 mL of solution is 2.88 mmHg at 25 C. Determine the molar mass of the protein. a) 246 g/mol b) 3.85 g/mol c) 2.93 *...
-
A solution containing 27.55 mg of an unknown protein per 25.0 mL solution was found to have an osmotic pressure of 3.22 torr at 25 C. What is the molar mass of the protein?
-
Calculate the osmotic pressure of a solution containing 18.75 mg of hemoglobin in 15.0 mL of solution at 25 C. The molar mass of hemoglobin is 6.5 * 10 4 g/mol.
-
A website that reviews recent movies lists 6 five-star films (the highest rating), 17 four-star films, 14 three-star films, 9 two-star films, and 4 one-star films. Make a frequency table for the data...
-
Nico Parts, Inc., produces electronic products with short life cycles (of less than two years). Development has to be rapid, and the profitability of the products is tied strongly to the ability to...
-
In 2019, Jasmine and Thomas, a married couple, had taxable income of $150,000. If they were to file separate tax returns, Jasmine would have reported taxable income of $140,000 and Thomas would have...
-
Gomez Co. had the following transactions in the last two months of its year ended December 31. Required 1. Prepare entries for these transactions under the method that records prepaid expenses as...
-
CVP analysis, income taxes The Express Banquet has two restaurants that are open 24-hours a day. Fixed costs for the two restaurants together total $459,000 per year. Service varies from a cup of...
-
5. Yankee Candle Company has two production departments, Forming and Finishing. The company uses a job-order costing system and computes a predetermined overhead rate in each production department....
-
This is a new manufacturing corporation that issued $50000 common stock for cash on the first day. All overhead expenses are paid immediately by cash. To make it easy, there are no other operating...
-
What is the heat of hydration ( Hhydration )? How does the enthalpy of solution depend on the relative magnitudes of Hsolute and Hhydration ?
-
Which of these aqueous solutions has the highest boiling point? a) 1.25 M C 6 H 12 O 6 b) 1.25 M KNO 3 c) 1.25 M Ca(NO 3 ) 2 d) None of the above (they all have the same boiling point)
-
At what stage in the development of a supply chain is Oak Hills, and what can be done to improve the supply chain?
-
A strain gauge transducer uses a Wheatstone bridge circuit to measure strain using resistance as shown in Figure Q4. The maximum permissible instrument gauge current is 0.25 A. (a) (b) R Figure Q4...
-
Describe at least four forces for change and four for the status quo. Then, outline the change(s) you want to see. Describe the end goal and the consequence of the change with one to two sentences....
-
Draw the diagram in one color for the first move and then another color for the second move. 1. Move 1: Translate 5 units right and 1 unit up. Move 2: Rotate 90 clockwise around the origin. List the...
-
QUESTION 3 Kindly refer to the article entitled " What is Digital Banking and How Will it Benefits Malaysians?" , Vulcan Post, 7 April 2020 below and answer the following questions:- (i) Define what...
-
Taking a look at the current real estate development environment, please read: Tishman Speyer announced that it has acquired The Eddy, a recently-constructed, high-rise apartment tower located on the...
-
Using Table 2.2, determine the number of covalent bonds that are possible for atoms of the following elements: germanium, phosphorus, selenium, and chlorine.
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
Stemming a chimney. A rock climber of mass 60 kg wants to make her way up the crack between two rocks as shown in Figure P4.14. The coefficient of friction between her shoes and the rock surface is...
-
Give an example in which the acceleration is perpendicular to the velocity.
-
For the system in Problem 12 and Figure P4.12, how large can m 2 be made without the system starting into motion? Data From Problem 12 Two blocks of mass m 1 = 45 kg and m 2 = 12 kg are connected by...
-
As of Nov 21/2020, the price-to-earnings ratio of Tesla's competitor is 38.77. Tesla's earnings per share are $0.56. Tesla has 985.5 million shares outstanding. Based on the competitor, what is the...
-
Nash Company exchanged equipment used in its manufacturing operations plus $4,020 in cash for similar equipment used in the operations of Tony LoBianco Company. The following information pertains to...
-
Suppose you want to with draw RM 5,000 at the end of five years and with draw RM 6,000 at the end of six years, leaving a zero balance in the account after the last withdrawal. If you can earn 5% on...

Study smarter with the SolutionInn App


