The structures of two isomers of heptane are shown. Which of these two compounds would you expect
Question:
The structures of two isomers of heptane are shown. Which of these two compounds would you expect to have the greater viscosity?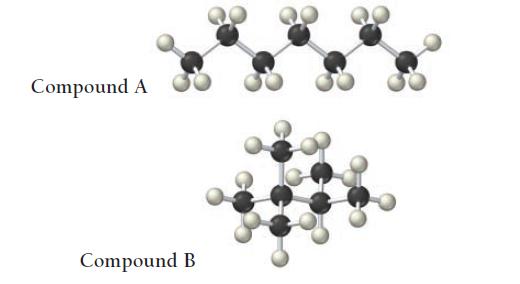
Transcribed Image Text:
Compound A Compound B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of these compounds would you expect to have the highest boiling point? Explain. [Section 24.4] CH3CH CH CH OH CHC=CH HCOCH
-
Which of the following compounds would you expect to have a dipole moment? If the molecule has a dipole moment, specify its direction. (b) H2O (c) CH4 (d) CH3Cl (e) CH2O (f) HCN
-
Which liquid would you expect to have a greater viscosity, water or diethyl ether? The structure of diethyl ether is shown in Problem 11.14?
-
ed The Engine Guys produces specialized engines for "snow climber buses. The company's normal monthly production volume is 2,500 engines, whereas its monthly production capacity is 5,000 engines. The...
-
The Supreme Court of State A ruled that, under the law of State A, pit bull owners must either keep their dogs fenced or pay damages to anyone bitten by the dogs. Assess the accuracy of the following...
-
Powersys is an electricity distribution company based in a large capital city. Its business is to manage the electricity assets, including poles, wires, and other equipment, that are used to deliver...
-
What ethical values or principles are involved in the lack of accurate reporting of cracks at the nuclear reactors in the late 1980s and 1990s?
-
Piere Imports uses the perpetual system in accounting for merchandise inventory and had the following transactions during the month of October. Prepare entries to record these transactions assuming...
-
The unadjusted pre-closing 12/31/23 account balances for the Mahoney Company are listed below: Net Sales $11,015 Net Purchases 8,991 Selling Expenses 430 Cash 564 Machines 6,424 Accumulated...
-
1. What is your assessment of the financial performance of Nelson Nurseries? 2. Do you agree with Christine Barton?s accounts-payable policy? 3. What explains the erosion of the cash balance? 4. What...
-
Explain why the viscosity of multigrade motor oils is less temperature-dependent than that of single-grade motor oils.
-
Water (a) wets some surfaces and beads up on others. Mercury (b), in contrast, beads up on almost all surfaces. Explain this difference. 1 (a) (b)
-
In The General Theory of Employment, Interest, and Money, John Maynard Keynes wrote: If the Treasury were to f ill old bottles with banknotes, bury them at suitable depths in disused coal mines which...
-
University Printers has two service departments (Maintenance and Personnel) and two operating departments (Printing and Developing). Management has decided to allocate maintenance costs on the basis...
-
In 2026, Blossom Company purchased the net assets of Ayayai Corporation for $2226400. On the date of the transaction, Ayayai had $607200 of liabilities. The fair value of Ayayai's assets when...
-
Financial statement data for the years ending December 31, 20Y3 and 20Y2, for Lawson Company follow: Sales Total assets: Beginning of year End of year 20Y3 $1,400,000 610,000 790,000 20Y2 $1,026,000...
-
$ 12,865 The Fiberglass Boat Company reported the following costs and expenses in May 2021: Factory utilities Direct labor $209,860 Depreciation on factory Sales staff salaries 134,520 equipment...
-
If the price of X is $26, the firm will (a) shut down in the short run. Wrong. The company will produce if Price>AVC at any level of Q. If Price>AVC the company makes some contribution to fixed...
-
Draw a reaction energy diagram for a one-step reaction that is very slow and slightly endothermic.
-
In Problem use absolute value on a graphing calculator to find the area between the curve and the x axis over the given interval. Find answers to two decimal places. y = x 3 ln x; 0.1 x 3.1
-
The conversion of triglycerides into biodiesel can be achieved in the presence of either catalytic acid or catalytic base. We have seen a mechanism for transesterification with catalytic acid. In...
-
A lecithin was hydrolyzed to yield two equivalents of myristic acid. (a) Draw the structure of the lecithin. (b) This compound is chiral, but only one enantiomer predominates in nature. Draw the...
-
A cephalin was hydrolyzed to yield one equivalent of palmitic acid and one equivalent of oleic acid. (a) Draw two possible structures of the cephalin. (b) If the phosphodiester was located at C2 of...
-
Winter Time Adventures is going to annual dividend if $2.61 a share on its common stock next week. This year, the company paid a dividend of $2.50 a share. The company adheres to a constant rate of...
-
Small Factory : -Regular time 8 hours per day. -1 hour daily lunch break. -25 working days per month. -50 workers. -Worker productivity 2.5 units per hour. -sold for $ 150 per unit. -cost of Labor...
-
$500 is invested for 7 years at 10 % p.a. simple interest. How much will the investment be worth after this period

Study smarter with the SolutionInn App


