Upon combustion, a 0.8233-g sample of a compound containing only carbon, hydrogen, and oxygen produces 2.445 g
Question:
Upon combustion, a 0.8233-g sample of a compound containing only carbon, hydrogen, and oxygen produces 2.445 g CO2 and 0.6003 g H2O. Find the empirical formula of the compound.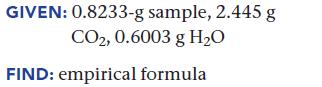
Transcribed Image Text:
GIVEN: 0.8233-g sample, 2.445 g CO2, 0.6003 g H₂O FIND: empirical formula
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
2445 g CO X 005556 1 mol CO 4401 g CO mol CO 06003 gHO ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound containing only carbon and hydrogen produces 113.49 g CO2 and 54.36 g H2O when combusted. What is the empirical formula of this compound? If it has a molar mass of 86.20 g/mol, what is its...
-
Write a program "three.c" which contains a function (named "three") that takes two arguments: a pointer to an int and a pointer to a char. The function should return a float. This function should...
-
Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO 2 and 0.901 g H 2 O. Find the empirical formula of the compound. GIVEN: 1.83 g CO, 0.901 g HO FIND: empirical formula
-
Selling price Variable costs: Direct materials Direct labour Variable manufacturing overhead Total variable cost Contribution margin Contribution margin ratio Contribution margin per labour hour O...
-
Kaleb Konstruction, Inc., has the following mutually exclusive projects available. The company has historically used a three-year cutoff for projects. The required return is 10 percent. (a) Calculate...
-
Olive Construction Company is determining whether it should submit a bid for a new shopping center. In the past, Olive's main competitor, Base Construction Company, has submitted bids 70% of the...
-
Do the advantages of ratio analysis outweigh the disadvantages? Discuss. L01
-
On April 15, Sanborn Company sold merchandise to Barr Company for $3,000 on terms of 2/10, n/30. Assume a return of merchandise on April 20 of $600 and collection in full on April 25. What is the...
-
Lok Co. reports net sales of $5,318,000 for Year 2 and $7,709,000 for Year 3. End-of-year balances for total assets are Year 1, $1,506,000; Year 2, $1,849,000; and Year 3, $1,934,000. (1) Compute...
-
A 40-m-long, 4-in. commercial steel pipe connects reservoirs A and Bas shown in Figure P4.1.6. Determine the minimum pressure (P0) that would keep the pressure head throughout the pipe positive....
-
What are functionalized hydrocarbons? Cite an example of a functionalized hydrocarbon.
-
What is the difference between an alkane, an alkene, and an alkyne?
-
What are the principal methods used to arrive at transfer prices? pg14
-
Calculate the base value or lump sum for each of the single and married filing jointly 2016 brackets given in Table 6.4. Table 6.4 ITABLE 6.4 Corporate Income Brackets and Tax Rates, 2015 Taxable...
-
Show that staged column diameter is proportional to (feed rate) \({ }^{1 / 2}\) and to \((1+\mathrm{L} / \mathrm{D})^{1 / 2}\).
-
An atmospheric column with 25 real stages is operating with a pressure drop of 0.6 in. of water per stage. Assume pressure drop in the condenser and the reboiler is \(1.2 \mathrm{in}\). of water...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A parabola with the distance between the directrix and focus 1 unit
-
Why is the Rosenblum case a particularly important case in auditor liability?
-
In mice, a dominant allele that causes a short tail is located on chromosome 2. On chromosome 3, a recessive allele causing droopy ears is 6 mu away from another recessive allele that causes a flaky...
-
Explain the buyers position in a typical negotiation for a business. Explain the sellers position. What tips would you offer a buyer about to begin negotiating the purchase of a business?
-
What is the physical basis for the experimental result that U is a function of V at constant T for a real gas? Under what conditions will U decrease as V increases?
-
Why didnt Joule change his experiment to make C surroundings /C system approx = 0.001 to increase the sensitivity of the apparatus?
-
Why does the relation C P > C V always hold for a gas? Can C P < C V be valid for a liquid?
-
Jarvis Company uses the total cost concept of applying the cont plus approach to product pricing. The costs and expenses of producing and selling 3.000 of Products follow Variable costs Direct...
-
plz help Match the form with its dotition B. Payback Method A. The sun role of return of a pract Hurdle Rate B. Measures the amount of time takes to recover the original investment Cute time value...
-
The following amortization and interest schedule reflects the issuance of 10-year bonds by Flint Corporation on January 1, 2014, and the subsequent interest payments and charges. The companys...

Study smarter with the SolutionInn App


