Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO 2 and 0.901 g
Question:
Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO2 and 0.901 g H2O. Find the empirical formula of the compound.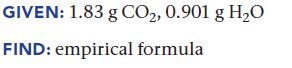
Transcribed Image Text:
GIVEN: 1.83 g CO₂, 0.901 g H₂O FIND: empirical formula
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
183 g CO X 1 mol CO 4401 g CO 00416 mol CO 0901 g HO X 1 mol HO 1802 ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound containing only carbon and hydrogen produces 113.49 g CO2 and 54.36 g H2O when combusted. What is the empirical formula of this compound? If it has a molar mass of 86.20 g/mol, what is its...
-
Write a program "three.c" which contains a function (named "three") that takes two arguments: a pointer to an int and a pointer to a char. The function should return a float. This function should...
-
A compound containing only carbon and hydrogen is 85.63% C by mass. Reaction of this compound with H2O produces a secondary alcohol as the major product and a primary alcohol as the minor product. If...
-
Burger Prince buys top-grade ground beef for $1.00 per pound. A large sign over the entrance guarantees that the meat is fresh daily. Any leftover meat is sold to the local high school cafeteria for...
-
Suppose you have a project with a payback period exactly equal to the life of the project. What do you know about the IRR of the project? Suppose that the payback period is never. What do you know...
-
Consider the following contingency table: What is the probability of a. event A'? b. event A and B? c. event A' and B'? d. event A' or B'? A 25 35
-
Understand the limitations of ratio analysis. L01
-
A storage tank acquired at the beginning of the fiscal year at a cost of $86,000 has an estimated residual value of $10,000 and an estimated useful life of eight years. Determine the following: (a)...
-
You plan on retiring in 2 5 years. In order to increase your retirement income, you open a retirement account today, and make a $ 2 0 , 0 0 0 deposit. In addition, you will deposit $ 5 , 0 0 0 every...
-
SC Consulting, a supply chain consulting firm, must decide on the location of its home offices. Its clients are located primarily in the 16 states listed in Table 5-5. There are four potential sites...
-
What is the difference between an alkane, an alkene, and an alkyne?
-
Which elements are normally present in organic compounds?
-
An experiment consists of rolling a pair of dodecahedral (12-sided) dice and recording their sum (the sides of each die are numbered from 1 to 12). The command shown in Figure A simulated 500 rolls...
-
Carol Lapaz owned a small company that sold boating equipment. The equipment was expensive, and a perpetual system was maintained for control purposes. Even so, lost, damaged, and stolen merchandise...
-
The following footnote related to accounting for inventory was taken from the 2008 annual report of Wal-Mart, Inc. Inventories The Company values inventories at the lower of cost or market as...
-
Plot the magnitude and phase of the frequency response of normalized n-th order lowpass Butterworth filters.
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
If two genes are more than 50 mu apart, how would you ever be able to show experimentally that they are located on the same chromosome?
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
Why is it reasonable to write dH C P dT + VdP for a liquid or solid sample?
-
For each of the following pairs of compounds, identify the higher boiling compound and justify your choice: a. b.
-
Refer to Figure 1.10 and explain why (U/V) T is generally small for a real gas. Figure 1.10 Ideal gas Real gas Tranlaition rv-0 (4)A
-
On May 1, Year 1, Benzs Sandwich Shop loaned $22,000 to Mark Henry for one year at 10 percent interest. Required a. What is Benzs interest income for Year 1? b. What is Benzs total amount of...
-
O $400 O 5000 QUESTION 15 Generally a partner contributing property to a partnership will not recognize any gain or loss on such contribution o True O False QUESTION 16 Which of the following kinds...
-
1. UNIS Let's start with an easy one! Which of the following wou with an easy one! Which of the following would most likely be a fixed cost for a Subway restaurant? a. Lettuce b. Fritos chips c....

Study smarter with the SolutionInn App


