Use the mass spectrum of lead to estimate the atomic mass of lead. Estimate the mass and
Question:
Use the mass spectrum of lead to estimate the atomic mass of lead. Estimate the mass and percent intensity values from the graph to three significant figures.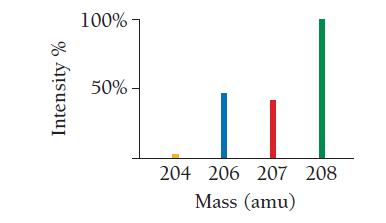
Transcribed Image Text:
Intensity % 100%- 50%- 204 206 207 208 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
o estimate the atomic mass of lead from the mass spectrum we can take the f...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the mass spectrum of mercury to estimate the atomic mass of mercury. Estimate the masses and percent intensity values from the graph to three significant figures. Intensity % 100% 50%- 196 198...
-
Mass spectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The...
-
The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 43.27% and an M + 1 peak with a relative intensity of 3.81%. How many carbon atoms are in the compound?
-
Show that = E[(m(X) - X'p)] B = argmind (b) = (E[XX']) E[Xm(X)] = [E[XX'])E[XY]. berk Hint: To show E[Xm(X)] = E[XY] use the law of iterated expectations. then
-
You are scheduled to receive $15,000 in two years. When you receive it, you will invest it for six more years at 8 percent per year. How much will you have in eight years?
-
What key ideas would you include in an e-mail message to persuade your congressional representative to support an issue that is important to you?
-
Three different bond issuances are listed here with interest payments made Semiannually. Bond Issuance Face Value Stated% A B C 100,000 400,000 600,000 Interest Rate 6% 8 6 Effective Interest Rate 6%...
-
Sala Co. is contemplating the replacement of an old machine with a new one. The following information has been gathered: If the old machine is replaced, it can be sold for $20,000. 1. Which of the...
-
Abigail is a manager at her company. The company just launched an initiative to improve its corporate citizenship practices. Abilgail is responsible for all but which of the following areas?...
-
You are the brand manager for your favorite brand of clothing, food, vehicle, or other consumer product. Write a one-page branding statement summarizing your brand for your company's VP of Marketing....
-
Naturally occurring iodine has an atomic mass of 126.9045 amu. A 12.3849 g sample of iodine is accidentally contaminated with an additional 1.00070 g of 129 I, a synthetic radioisotope of iodine used...
-
An a particle, 4 He 2+ , has a mass of 4.00151 amu. Find the value of its charge-to-mass ratio in C/kg.
-
The financial statements of P&G are presented in Appendix B. The companys complete annual report, including the notes to the financial statements, is available online. Instructions Refer to P&Gs 2017...
-
QUESTION: "When companies go beyond development and facilitate growth in the flow of work, they are much more likely to build skills for the future." Provide recommendations to organization looking...
-
How do pilots interpret sustainable practices to improve workforce development and diversity, increase quality and efficiency while reducing costs in aviation and aerospace operations.
-
Develop a program model for training and development for nee line middle managers in healthcare organizations.
-
Describe each of the following in relation to policy development and implementation in the early childhood sector Codes ofpractice Duty ofcare Humanrights Privacy, confidentiality anddisclosure...
-
Martinez Ltd. had the following 2023 income statement data: Revenues $110,000 Expenses 50,900 $59.100 In 2023, Martinez had the following activity in selected accounts: Accounts Receivable Allowance...
-
The Mic2 gene in humans is present on both the X and Y chromosome. Let's suppose the Mk2 gene exists in a dominant Mic2 allele, which results in normal surface antigen, and a recessive mic2 allele,...
-
The Place-Plus real estate development firm in Problem 24 is dissatisfied with the economists estimate of the probabilities of future interest rate movement, so it is considering having a financial...
-
Malonic acid has two acidic protons: The pKa of the first proton (pK 1 ) is measured to be 2.8, while the pK a of the second proton (pK 2 ) is measured to be 5.7. (a) Explain why the first proton is...
-
Identify a systematic (IUPAC) name for each of the following compounds a. b. c. d. (e) CH 3 (CH 2 ) 4 CO 2 H (f) CH 3 (CH 2 ) 3 COCl (g) CH 3 (CH 2 ) 4 CONH 2 O: NH2
-
Identify the common name for each of the following compounds: a. b. c. d.
-
gnment CALCULATOR FLEEN PRINTER VERSION BACK NEXT Multiple Choice Question 142 During January 2017, its or month of operation, Osborn Enterprises earned net income of 6800 and paid dividends to the...
-
x Company is considering buying a part next year that they currently make. This year's production costs for 3,400 units were as follows: Direct materials Direct labor Variable overhead Fixed overhead...
-
Package Corporation acquired 90 percent ownership of Sack Grain Company on January 1, 20X4, for $118,800 when the fair value of Sack's net assets was $19,000 higher than its $113,000 book value. The...

Study smarter with the SolutionInn App


