Use the mass spectrum of mercury to estimate the atomic mass of mercury. Estimate the masses and
Question:
Use the mass spectrum of mercury to estimate the atomic mass of mercury. Estimate the masses and percent intensity values from the graph to three significant figures.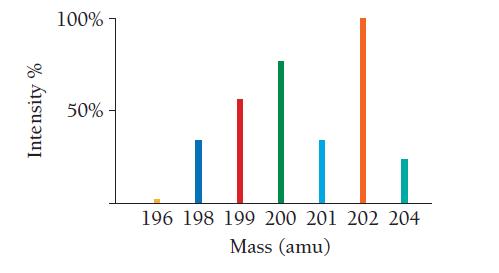
Transcribed Image Text:
Intensity % 100% 50%- 196 198 199 200 201 202 204 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The mass spectrum of mercury shows two peaksone at 199 amu and one at 201 amuThe peak at 199 am...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the mass spectrum of lead to estimate the atomic mass of lead. Estimate the mass and percent intensity values from the graph to three significant figures. Intensity % 100%- 50%- 204 206 207 208...
-
The masses of the naturally occurring mercury isotopes are 196 Hg, 195.9658 u; 198 Hg, 197.9668 u; 199 Hg, 198.9683 u; 200 Hg, 199.9683 u; 201 Hg, 200.9703 u; 202 Hg, 201.9706 u; and 204 Hg, 203.9735...
-
Mass spectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The...
-
Suppose that you just purchased 200 shares of Talk&Tell stock for $60 per share. a. If the initial margin requirement is 71.00%, how much money must you borrow? b. Construct the balance sheet...
-
You have $7,000 to deposit. Regency Bank offers 12 percent per year compounded monthly (1 percent per month), while King Bank offers 12 percent but will only compound annually. How much will your...
-
5. From the following particulars of M/s Swapnil enterprises, prepare a Bank reconciliation statement: (1) Bank overdraft as per Pass Book as on 31st March, 2021 was * 8,800 (2) Cheques deposited in...
-
Tingham Village issued 500 five-year bonds on July 1, 1997. The interest payments are due semiannually (January 1 and July 1) at an annual rate of 6 percent. The effective interest rate on the bonds...
-
In 2015, a city opens a municipal landll, which it will account for in an enterprise fund. It estimates capacity to be 6 million cubic feet and usable life to be 20 years. To close the landll, the...
-
A partial amortization schedule for a 10 year note payable issued on January 1, Year 1. is shown next Accounting Principal Balance Applied to Applied to Period January 1 Cash Payment Interest Year 1...
-
The box plot below shows the amount spent for books and supplies per year by students at four year public colleges. a. Estimate the median amount spent. b. Estimate the first and third quartiles for...
-
Fill in the blanks to complete the table. Symbol Z A Si 14 S- 2+ Cu+ 15 32 Number Number Number of p of e of n 14 14 15 34 16 Charge 2- 2+
-
Naturally occurring iodine has an atomic mass of 126.9045 amu. A 12.3849 g sample of iodine is accidentally contaminated with an additional 1.00070 g of 129 I, a synthetic radioisotope of iodine used...
-
A channel with square cross section is to carry \(20 \mathrm{~m}^{3} / \mathrm{s}\) of water at normal depth on a slope of 0.003 . Compare the dimensions of the channel required for (a) concrete (b)...
-
Problem 10-19 (Algo) Internal Business Process Performance Measures [LO10-3] Tombro Industries is in the process of automating one of its plants and developing a flexible manufacturing system. The...
-
During 2023, Emily worked as a financial controller for Vector Industries (VI) and earned a salary of $150,000. VI downsized its office space after the pandemic, and adopted a hybrid working policy...
-
Transactions for Buyer and Seller Shore Co. sold merchandise to Blue Star Co. on account, $110,400, terms FOB shipping point, 2/10, n/30. The cost of the goods sold is $66,240. Shore paid freight of...
-
The following information relates to the Jasmine Company for the upcoming year, based on 418,000 units: Sales Cost of goods sold Gross margin Operating expenses Operating profits 6,688,000 Amount $...
-
Sherri owes $5,500 on her credit card. The card has an APR of 14.9 percent. Sherri has decided not to charge any additional purchases because she wants to get this debt paid in full. A) How much...
-
As discussed in this chapter, comb morphology in chickens is governed by a gene interaction. Two walnut comb chickens were crossed to each other. They produced only walnut comb and rose comb...
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
There are five constitutional isomers with molecular formula C 4 H 8 . One of the isomers exhibits a particularly strong signal at M15 in its mass spectrum. Identify this isomer, and explain why the...
-
There are four isomers with molecular formula C 4 H 9 Cl. Only one of these isomers (compound A) has a chirality center. When compound A is treated with sodium ethoxide, three products are formed:...
-
Identify the number of Ï electrons in each of the following compounds. a. b. c. d. e. N'
-
Both answers please Problem - 7 Akimora Dairy began operations on April 1, 2015, with purchase of 250 miking cows for 18.500.000. It has completed the first month of operations and has the following...
-
need help answering questions 3. What is the deferral adjustment associated with deferred revenue? a. Dr. Deferred Revenue (liability - BS), Cr. Revenue (revenue - IS) 4. What is the deferral...
-
Hosmer Corporation issued $190,000 par value, 6%, 4-year bonds (i.e., there were 190 of $1,000 par value bonds in the issue). Interest is payable semiannually each January 1 and July 1 with the first...

Study smarter with the SolutionInn App


