We can obtain titanium metal from its oxide according to the following balanced equation: When 28.6 kg
Question:
We can obtain titanium metal from its oxide according to the following balanced equation:![]()
When 28.6 kg of C reacts with 88.2 kg of TiO2, 42.8 kg of Ti is produced. Find the limiting reactant, theoretical yield (in kg), and percent yield.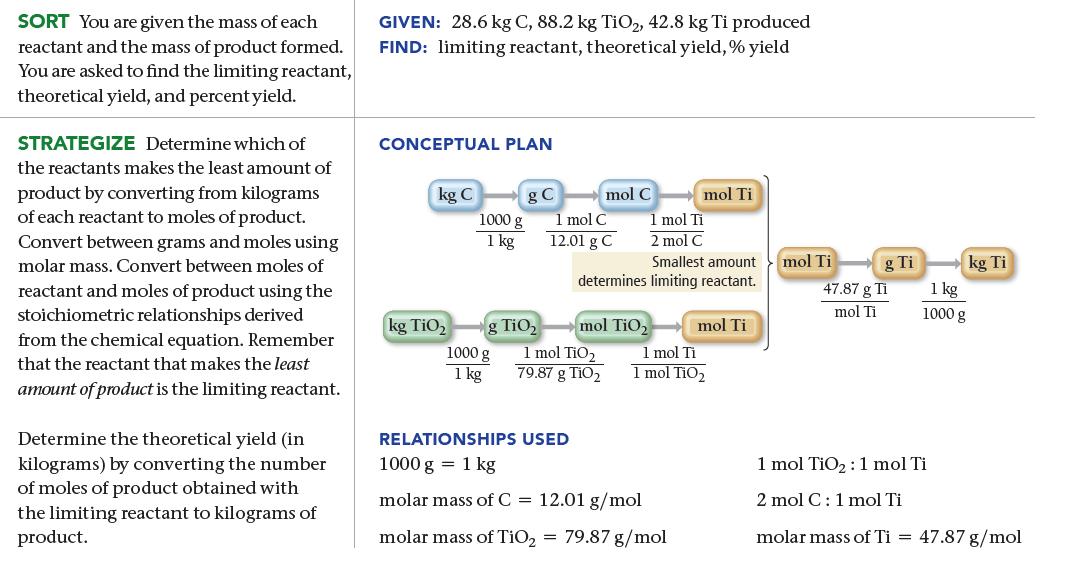
Transcribed Image Text:
TiO₂ (s) + 2 C(s) Ti(s) + 2 CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
286 kg ex Limiting reactant 882 kgFiO X 1000g 1 kg 1000 X 1 kg 1 mot C ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The main reaction of a charcoal grill is C(s) + O2(g) CO2(g). Which of the statements below are incorrect? Why? a. 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of CO2. b. 1...
-
Twelve years ago, Birch Ltd. (BL) borrowed $480,000 from Oak Trust Inc. (OTI). The 12- year, 10% note is due on todays date, December 31, 2020. The note was originally issued at par. BL is unable to...
-
What are some techniques of good writers? Which ones do you use regularly?
-
Rick Darman, the owner-president of Computer Services, is unfamiliar with the statement of cash flows that you, as his accountant, prepared. He asks for further explanation. Instructions Write him a...
-
Show that COPHP = COPR + 1 when both the heat pump and the refrigerator have the same QL and QH values.
-
Explain how the real option value of an investment is derived from ROV analysis.
-
The following data (dollar amounts in millions) are from the financial statements of Valley Corporation: Requirement 1. Complete the folloeing condensed income statement, Report amounts to the...
-
At the beginning of the current season on April 1, the ledger of Sunland Pro Shop showed Cash $2,940; Inventory $3,500; and Common Stock $6,440. The following transactions were completed during April...
-
Jamie Lee Jackson, age 26, is in her last semester of college and is anxiously waiting for graduation day that is just around the corner! She still works part-time as a bakery clerk, has been...
-
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: Suppose that 5 mol NO 2 and 1 mol H 2 O combine and react completely. How many moles of the...
-
Sulfur and fluorine react to form sulfur hexafluoride: If 50.0 g S is allowed to react as completely as possible with 105.0 g F 2 , what mass of the excess reactant is left? a) 20.5 g S b) 45.7 g F 2...
-
Find the general solution of the differential equation. dr/ds = 4/9 r
-
How do changing geopolitical landscapes, such as shifting alliances and emerging power centers, influence conflict resolution strategies, and what adjustments are necessary to address new global...
-
50 21 2. Determine the inclination and period of the satellite which produced the ground trace below. Show all calculations. Suteite 17 11-140-130-120-110 tonn an 20 6058 am 50 210 0 10 20 30 50 60...
-
This activity aims to provide practical experience in preparing tax forms related to business income and depreciation. It emphasizes the importance of accurate reporting and adherence to tax...
-
How do intersectionality and identity salience intersect within the framework of diversity and inclusion initiatives, and what strategies can organizations employ to address these complexities ?
-
(a) -2-3 3. Evaluate the following determinants: 3 5 (b) |- 58 -8 -2 4 312 4 3 0 (c) 2 245 (d) 3 1 2 245 5 -1 -4
-
A human gene called the -globin gene encodes a polypeptide that functions as a subunit of the protein known as hemoglobin. Hemoglobin is found within red blood cells; it carries oxygen. In human...
-
Walker, Inc., is an all-equity firm. The cost of the company's equity is currently 11.4 percent and the risk-free.rate is 3.3 percent. The company is currently considering a project that will cost...
-
Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and b in Table 7.4. Table 7.4 RedlichKwong van der...
-
Calculate the RedlichKwong parameters of fluorine from the values of the critical constants and compare your results with the values for a and b in Table 7.4. Table 7.4 RedlichKwong van der Waals b...
-
Calculate the critical volume for ethane using the data for T c and P c in Table 7.2 (see Appendix B, Data Tables) assuming a.The ideal gas equation of state b. The van der Waals equation of state....
-
Exercise 4-50 Theory of Constraints (LO 4-5) CompDesk, Inc., makes a single model of an ergonomic desk (with chair) for computer usage. The desk is manufactured in building 1, and the chair is...
-
In a capital account, explain which side (debit or credit) will decrease and which side will increase. Provide an example (just make one up) of a transaction with a T-account for each side of the...
-
How much sales revenue must ABC Catering generate in order to break

Study smarter with the SolutionInn App


