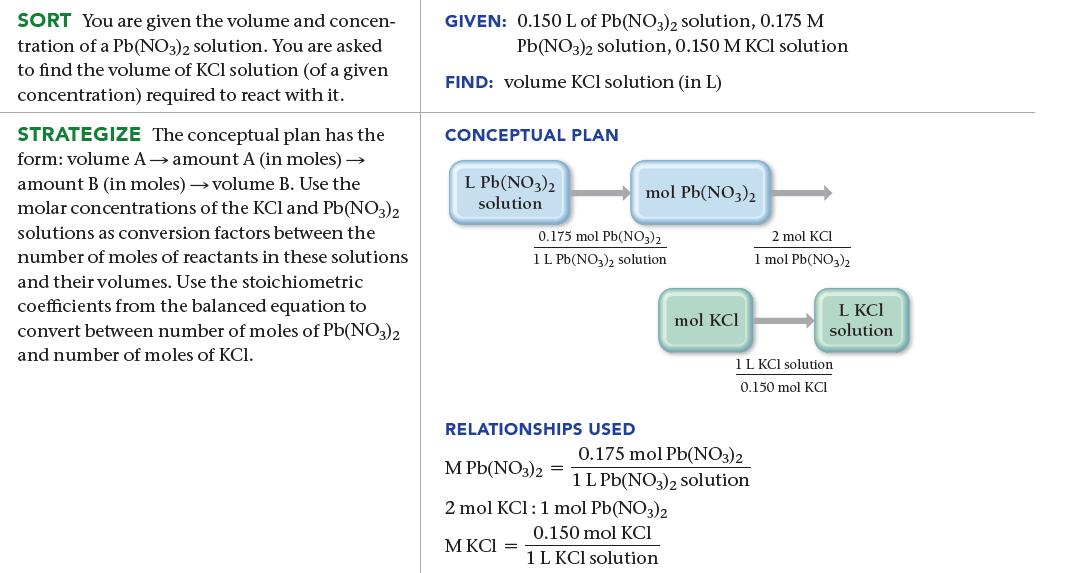
What volume (in L) of a 0.150 M KCl solution will completely react with 0.150 L of
Question:
What volume (in L) of a 0.150 M KCl solution will completely react with 0.150 L of a 0.175 M Pb(NO3)2 solution according to the following balanced chemical equation?
![]()
Transcribed Image Text:
2 KCl(aq) + Pb(NO3)2(aq) PbCl₂(s) + 2 KNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
0150 L PbNO32 solution X 2 mol KCI 1 mol PbNO32 0175 mol PbNO32 1 LPbNO32 solution 1 L KCl solu...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Twelve years ago, Birch Ltd. (BL) borrowed $480,000 from Oak Trust Inc. (OTI). The 12- year, 10% note is due on todays date, December 31, 2020. The note was originally issued at par. BL is unable to...
-
What volume of 0.512 M NaOH will react with 17.9 g of H2C2O4(s) according to the following chemical equation? H2C2O4(s) + 2NaOH(aq) ( Na2C2O4(aq) + 2H2O()
-
In the Young's double slit experiment, interference fringes are formed using Na-light of 589 nm and 589.5 nm. Obtain the region on the screen where the fringe pattern will disappear. You may assume...
-
It's Yours Manufacturing wishes to maintain a growth rate of 8 percent a year, a debt-equity ratio of 0.45, and a dividend payout ratio of 25 percent. The ratio of total assets to sales is constant...
-
Herb lives in Calgary. When it was time to shop for the best interest rate on a mortgage, Herb checked out interest rates at all of the major lending institutions. Just to be on the safe side, for...
-
What is off-balance-sheet financing? Why might a company structure a lease so that it is considered an operating lease instead of a capital lease?
-
Never-Die Battery manufactures batteries for industrial and consumer use. The company purchased a commercial package policy (CPP) to cover its property exposures. In addition to common policy...
-
(5 6. A company reported the following data related to its ending inventory: Product Units Available Cost Market Yellow 88 $ 15 $ 10 Blue 144 14 18 Black 222 12 20 Red 106 6 5 Orange 175 22 26 White...
-
In this mini-case, you will complete the test of details on accounts receivable for the 2019 audit of EarthWear Clothiers, Inc. The principal test of detail involves sending "confirmations" or...
-
Explain how a strong electrolyte, a weak electrolyte, and a nonelectrolyte differ.
-
The image shown at the far right represents a small ANSWER NOW! volume within 500 mL of aqueous ethanol (CH 3 CH 2 OH) solution. (The water molecules have been omitted for clarity.) Which of the...
-
On January 1, 2020, the City of Graf pays $60,000 for a work of art to display in the local library. The city will take appropriate measures to protect and preserve the piece. However, if the work is...
-
Unit 10 Seminar This week's Seminar will address leadership development and succession. What are two major components of leadership self-development? Development occurs through education,...
-
Do Home Depot and Lowe's disclose research and development expense and how much - what is the percentage of such to net income? See the links for Home Depot and Lowes 10-K Lowes 10-K...
-
Market Analysis: a. Target Market Development - identify and describe your market segments, product need, product awareness, consumer buying/shopping habits, product use. How does the culture of the...
-
what is the research and development for Netflix in the Philippines? Please Elaborate and give statistic or research about it.
-
Explain fire development within a compartment from the incipient stage to the decay stage. Make sure you cover each phase in detail. Make sure to use correct terminology. The purpose of this...
-
In the pedigree shown here for a trait determined by a single gene (affected individuals are shown in black), state whether it would be possible for the trait to be inherited in each of the following...
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
Predict the major product(s) for each of the following reactions: (a) (b) (c) (d) (e) (f) CH NH2 [H*] (-H20) 1) PhMgBr 2) H20
-
Identify the starting materials needed to make each of the following acetals: (a) (b) (c) OEt
-
Using ethanol as your only source of carbon atoms, design a synthesis for the following compound:
-
600 ? ? ? PA3. LO 3.2 Fill in the missing amounts for the four companies. Each case is independent of the others. Assume that only one product is being sold by each company. Company A Company B...
-
Question 2 : Cost V olum e - Profit (3. 0 ) Zan Bun is the sole producer of cakes in the Changlun region. The company only produces one type of cake, and it is sold at $10 each. The variable cost for...
-
39 40 Which of the following would result in an end-of-period adjustment for an accrued expense? Salaries owed to employees but not yet paid. Fees earned but not yet received. Equipment that...

Study smarter with the SolutionInn App


