The image shown at the far right represents a small ANSWER NOW! volume within 500 mL of
Question:
The image shown at the far right represents a small ANSWER NOW! volume within 500 mL of aqueous ethanol (CH3CH2OH) solution. (The water molecules have been omitted for clarity.)
Which of the following images best represents the same volume of the solution after we add an additional 500 mL of water?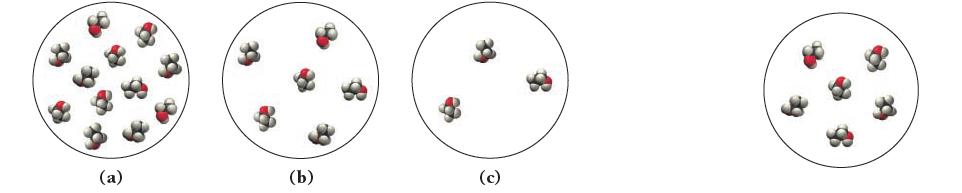
Transcribed Image Text:
(a) (b) 32 (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
c Since the volume has d...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following diagrams represent aqueous solutions of three acids, HX, HY, and HZ. The water molecules have been omitted for clarity, and the hydrated proton is represented as H+ rather than H3O+....
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The following diagrams represent aqueous solutions of two monoprotic acids, HA (A = X or Y). The water molecules have been omitted for clarity. (a) Which is the stronger acid, HX or HY? (b)Which is...
-
Research serves as a guide by which to study, describe, and quantify an array of social and physical phenomenon. Theory, which is best described as a set of propositions or hypotheses that specify...
-
After calculating the ratios for Smolira Golf, you have uncovered the following industry ratios for 2008: How is Smolira Golf performing based on these ratios? Lower Quartile: Median Upper Quartile...
-
Two economies, Hare and Tortoise, each start with a real GDP per person of $5000 in 1950. Real GDP per person grows 3% a year in Hare and 1% a year in Tortoise. In the year 2000, what will be real...
-
Explain the methods used to account for capital leases. How is the lease obligation amor tized over the life of the lease?
-
Potter Company has installed a JIT purchasing and manufacturing system and is using back ush accounting for its cost flows. It currently uses a two-trigger approach with the purchase of materials as...
-
Consider the following financial data for Salesforce, Inc.: US 10-Year T-Bond Yield = .63% Market Risk Premium = 6.25% Tax Rate = 21% Also the following data for Salesforce, Inc. : Stock Price =...
-
At time t = 0, the baseball player releases a ball with the initial conditions shown in the figure. Determine the quantities r, r, r, , , and , all relative to the x-y coordinate system shown, at...
-
What volume (in L) of a 0.150 M KCl solution will completely react with 0.150 L of a 0.175 M Pb(NO 3 ) 2 solution according to the following balanced chemical equation? 2 KCl(aq) + Pb(NO3)2(aq)...
-
What mass (in grams) of Mg(NO 3 ) 2 is present in 145 mL of a 0.150 M solution of Mg(NO 3 ) 2 ? a) 3.23 g b) 0.022 g c) 1.88 g d) 143 g
-
Sketch the following sets of points. {(r, ): 4 r 2 9}
-
Steve was the athletic trainer at John F. Kennedy High School. One of his responsibilities was to coordinate the annual physical examinations for all students planning to participate in...
-
* Make a well-researched analysis of the factors leading to the failures in (a) NASAs Apollo 18, 19 and 20 programs, and (b) Ford Motors in India in 2021. * Discuss in your paper various ways in...
-
1. What factors need to be in place for a service like the Dabbawallahs to function effectively? 2. What are the economics of the Dabbawallahs' meal distribution network (i.e., operational model)? Is...
-
The system archetype that might be in control of the case study is growth and under-investment. I chose this because the organization was small and they were trying to grow their organization by...
-
Was the crisis response and communication effective? Compare and contrast alternative digital communication efforts and provide alternative recommendations. Crisis One Staples is an office store...
-
The trait of hen- versus cock-feathering is a sex-limited trait controlled by a single gene. Females always exhibit hen-feathering as do HH and Hh males. Only hh males show cock-feathering. Starting...
-
Explain five different cases of income exempt from tax with clear examples.
-
Propose an efficient synthesis for each of the following transformations: (a) (b)
-
The compound below is believed to be a wasp pheromone. Draw the major product formed when this compound is hydrolyzed in aqueous acid:
-
Propose an efficient synthesis for each of the following transformations: (a) (b) (c) OMe Meo Meo Br Br
-
please help and show work thanks. Current Attempt in Progress Cullumber ProSystems needs a new signal conditioner module for a Large process control system it is designing. Current market conditions...
-
Required: 1. How many jobs must GTC average each month to break even? jobs per month 2. What is the operating income for GTC in a month with 89 jobs? Enter a net loss as a negative amount. $ What is...
-
Warren owns and manages a small business, which sells pet supplies to high street shops and market traders. Warren sells to customers on credit at 30 days credit terms. (Warren manages all aspects of...

Study smarter with the SolutionInn App


