Which of these two reactions would you expect to have the smaller orientation factor? Explain. a. O(g)
Question:
Which of these two reactions would you expect to have the smaller orientation factor? Explain.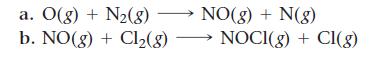
Transcribed Image Text:
a. O(g) + N₂(8) NO(g) + N(g) b. NO(g) + Cl₂(g) → NOCI(g) + Cl(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
The orientation factor or steric factor is a term used in collision theory to account for the probab...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider two hypothetical elements, W and Z. Element W has an electron affinity of 300 kJ/mol, and element Z has an electron affinity of 75 kJ/mol. a. If you have a W ion and a Z ion, from which ion...
-
Which of the following reactions would you expect to have the larger rate at room temperature? Why? (Hint: Think of which would have the lower activation energy.) 2Ce4+(aq) + Hg22+(aq) 2Ce3+(aq) +...
-
Consider these two gas-phase reactions: a. AA(g) + BB(g) 2 AB( g) b. AB(g) + CD(g) AC(g) + BD(g) If the reactions have identical activation barriers and are carried out under the same conditions,...
-
Write a program that takes an integer command-line argument n and prints all the positive powers of 2 less than or equal to n. Make sure that your program works properly for all values of n.
-
Langdon Company produced 9,000 units during the past year, but only 8,200 of the units were sold. The following additional information is also available. Direct materials used .......... $79,000...
-
The Akais just finished calculating their taxable income for their 2014 joint federal income tax return. It totaled \($68,750\) and showed no tax credits. Just prior to filing their return, the Akais...
-
Name and describe three order-picking systems.
-
A fast-food franchise is considering operating a drive-up window food-service operation. Assume that customer arrivals follow a Poisson probability distribution, with an arrival rate of 24 cars per...
-
a
-
The proposed mechanism for the formation of hydrogen bromide can be written in a simplified form as: What rate law corresponds to this mechanism? Br(g) k 2Br(g) Br(g) + H(g) H(g) + Br(g) k3 HBr(g) +...
-
If a temperature increase from 20.0 C to 35.0 C triples the rate constant for a reaction, what is the value of the activation barrier for the reaction?
-
Show that the equation represents a circle and find the center and radius. x 2 + y 2 + x = 0
-
Is Time Running Out for Bed Bath & Beyond case study and answer following questions: 3-13 analyze bed bath & beyond using the competitive forces and value chain models. 3-14 define the problem faced...
-
Marcus expresses an interest in learning more about Katie's job position, telling her that he hopes to be in the position himself one day. Katie decides to take Marcus under her wing and teach him...
-
Losing to a Weaker Foe What began as a heavily conventional military campaign to unseat the regime of Saddam Hussein had become a bitter, unconventional struggle against frustrated Sunnis who...
-
Question A4 (12 marks) Comfort Dance Corporation (CDC) is a major distributor of dance shoes. All sales are on terms 2/10, n/30. CDC uses a perpetual inventory system. The March opening balance in...
-
7 A car rental company is interested in improving the customer experience. A data professional fixes typos and inaccuracies from a dataset containing feedback and ratings. They also verify and share...
-
Assuming that the load impedance is to be purely resistive, what load should be connected to terminals a-b of the circuits in Fig. 11.52 so that the maximum power is transferred to the load? 100 10...
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
Design a problem to help other students better understand obtaining the Fourier series from a periodic function. A periodic function is defined over its period as Find the Fourier series of h(t). (10...
-
Using Fig. 17.51 , design a problem to help other students better understand how to determine the exponential Fourier series from a periodic wave shape. Obtain the exponential Fourier series of the...
-
Find the trigonometric Fourier series for 7.5 0
-
5. The cost of retained earnings the required rate of If a firm cannot invest retained earnings to earn a rate of return return on retained earnings, it should return those funds to its st less than...
-
How much will be the loss from bad debts under new credit terms of 3/10 net, if the cost of capital is 15% and the unpaid accounts are written off after 60 days?
-
You need to accumulate $10,000. To do so, you plan to make deposits of $1,100 per year, with the first payment being made a year from today, in a bank account that pays 7 percent annual interest....

Study smarter with the SolutionInn App


