Write balanced complete ionic and net ionic equations for each reaction. a. KSO4(aq) b. NH4Cl(aq) c. AgNO3(aq)
Question:
Write balanced complete ionic and net ionic equations for each reaction.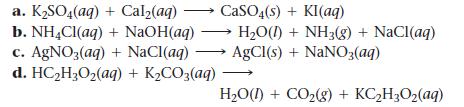
Transcribed Image Text:
a. K₂SO4(aq) b. NH4Cl(aq) c. AgNO3(aq) d. HC₂H₂O₂(aq) + K₂CO3(aq) + Cal₂(aq) + NaOH(aq) + NaCl(aq) CaSO4(s) + KI(aq) H₂O(1) + NH3(g) + NaCl(aq) AgCl(s) + NaNO3(aq) H₂O(1) + CO₂(g) + KC₂H₂O₂(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
here are the balanced complete ionic and net ionic equations for ...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HI(aq) + RbOH(aq) b. HCHO(aq) + NaOH(aq) c. HCHO(aq) + LiOH(aq)
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HBr(aq) + NaOH(aq) b. HF(aq) + NaOH(aq) C. HCHO(aq) + RbOH(aq)
-
Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq) HO(1)+ LiCI(aq) b. Cas(aq) + CuCl(aq) CuS(s) + CaCl(aq) c. NaOH(aq) + HCHO(aq) d. Na3PO4(aq) + NiCl(aq)...
-
What Codification citation indicates how unrealized gains or losses from changes in fair value affect net income when investments are classified as available-for-sale?
-
The Mark Goodwin Resort is an elegant summer resort located in a remote mountain setting. Guests visiting the resort can fish, hike, go horseback riding, swim in one of three hotel pools, or simply...
-
Cameron Oil Company, a successful efforts company, has capitalized costs on Property R, Property S, and Property T as of 12/31/2018 as follows: Cameron has no other capitalized costs. The properties...
-
What short-term tactics can help provide Bernice with more time to train? How, over the longer term, can Bernice learn how to be a more effective trainer?
-
At December 31, 2011, Hemington Company had 320,000 shares of common stock outstanding. Hemington sold 80,000 shares on October 1, 2012. Net income for 2012 was $1,985,000; the income tax rate was...
-
help pls Problem 19-01A Midlands Inc. had a bad year in 2019. For the first time in its history, it operated at a loss. The company's income statement showed the following results from selling 79,000...
-
Once upon a time many, many years ago, there lived a feudal landlord in a small province of Western Europe. The landlord, Baron Coburg, lived in a castle high on a hill. He was responsible for the...
-
Mercury(I) ions (Hg 2 2 + ) can be removed from solution by precipitation with Cl . Suppose that a solution contains aqueous Hg 2 (NO 3 ) 2 . Write complete ionic and net ionic equations for the...
-
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write NO REACTION. a. Sodium chloride and lead(II)...
-
Kays Auto Products budgeted sales of 10,000 units of product B, assuming that the company would have 20 percent of 50,000 units sold in a particular market. The actual results were 9,000 units, based...
-
Suppose youre applying a simulated annealing algorithm to a certain problem, where T is the parameter that measures the tendency to accept the current candidate to be the next trial solution. You...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Assume, further, that the acquisition was consummated on October 1, 2024, as described above. However, by the end of 2025, Ayayai was concerned that the fair values of one or both of the acquired...
-
You have been asked to prepare a brief presentation on a criminological topic or issue of interest to you. Go to the Bureau of Justice Statistics (BJS) Publications & Products Overview page (See link...
-
Sparta Fashions owns four clothing stores, where it sells a wide range of women's fashions, from casual attire to formal wear. In addition, it rents formal wear and gowns for special occasions. At...
-
A large plate is fabricated from a steel alloy that has a plane strain fracture toughness of 55 MPa m (50 ksi in.). If, during service use, the plate is exposed to a tensile stress of 200 MPa (29,000...
-
Compare and contrast debt financing and equity financing as ways of starting a new business. Does one have an overall advantage over the other? What situation is more favorable to the use of debt...
-
The maximum pressure that can be developed for a certain fluid power cylinder is 5000 psi. Compute the required diameter for the piston if the cylinder must exert a force of 20 000 lb.
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
A line of fluid power cylinders has a range of diameters in 1.00-in increments from 1.00 to 8.00 in. Compute the force that could be exerted by each cylinder with a fluid pressure of 500 psi. Draw a...
-
Suppose an investment is equally likely to have a 37.4% return or a -20% return. The total volatility of returns is closest to: Select one: a. 20.29% b. 28.70% c. 40.59% d. 8.24%
-
Discuss what determines whether a dwelling unit is treated as a residence or a non-residence for tax purposes. What are the ownership and use requirements a taxpayer must meet to qualify for the...
-
Oliver plans to invest $24,000 for 6.5 years. Wells Fargo offered him the following rates below. Which Wells Fargo rate should he accept so that he will have the largest future value? 4 percent...

Study smarter with the SolutionInn App


