Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq) HO(1)+ LiCI(aq)
Question:
Write balanced complete ionic and net ionic equations for each reaction.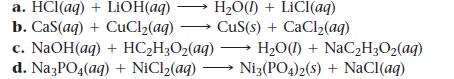
Transcribed Image Text:
a. HCl(aq) + LiOH(aq) H₂O(1)+ LiCI(aq) b. Cas(aq) + CuCl₂(aq) → CuS(s) + CaCl₂(aq) c. NaOH(aq) + HC₂H₂O₂(aq) d. Na3PO4(aq) + NiCl₂(aq) - H₂O() + NaC₂H₂O₂(aq) Ni3(PO4)2(s) + NaCl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Complete H aq Claq Lit aq OHaq Net Haq OHaq HO1 Liaq Claq HO1 b ...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HI(aq) + RbOH(aq) b. HCHO(aq) + NaOH(aq) c. HCHO(aq) + LiOH(aq)
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HBr(aq) + NaOH(aq) b. HF(aq) + NaOH(aq) C. HCHO(aq) + RbOH(aq)
-
Write balanced complete ionic and net ionic equations for each reaction. a. KSO4(aq) b. NH4Cl(aq) c. AgNO3(aq) d. HCHO(aq) + KCO3(aq) + Cal(aq) + NaOH(aq) + NaCl(aq) CaSO4(s) + KI(aq) HO(1) + NH3(g)...
-
Should courts ever enforce illegal contracts? If illegal contracts are void as a matter of law, what is the court enforcing? If courts will use equity powers or other roundabout ways to enforce...
-
Download a copy of the ACFE Compensation Guide for Anti-fraud Professionals, which is available at no charge at: www.acfe.com/documents/2008-comp-guide.pdf. Based on this resource, answer the...
-
Marvel Oil Corporation uses the full cost method of accounting. The following costs are incurred during 2019: During 2020, the following costs were incurred: Delay rentals were paid on Lease A,...
-
What types of training resources would best help Bernice as she attempts to train Lovi-Ann?
-
David Cheung is an operations manager for a large manufacturer. He earned $68,500 in 2012 and plans to contribute the maximum allowed to the firms 401(k) plan. Assuming that David is in the 25...
-
Branson paid $543,800 cash for all of the outstanding common stock of Wolfpack, Inc, on January 1, 2020. On that date, the subsidiary had a book value of $401.000 (common stock of $200,000 and...
-
Use conditional proof and the eighteen rules of inference to derive the conclusions of the following symbolized arguments. Having done so, attempt to derive the conclusions without using conditional...
-
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write NO REACTION. a. Sodium chloride and lead(II)...
-
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write NO REACTION. a. Potassium carbonate and...
-
What is electronic data interchange? List three types of data that a manufacturer and supplier might exchange electronically.
-
Dr. Stanley and his staff are attempting to utilize effective ways to both increase the revenue for the practice and allow patients to schedule visits without a lengthy delay. Which of the following...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Some aircraft component is fabricated from an aluminum alloy that has a plane strain fracture toughness of 35 MPa m (31.9 ksi in.). It has been determined that fracture results at a stress of 250 MPa...
-
Difference between truncate & delete
-
Repeat Problem 1.63 but change the length of the cylinder to 10.0 in. Compare the results. Repeat Problem A measure of the stiffness of a linear actuator system is the amount of force required to...
-
Repeat Problem 1.63 but change the cylinder diameter to 2.00 in. Compare the results. Repeat Problem A measure of the stiffness of a linear actuator system is the amount of force required to cause a...
-
Calculate the mass of a can of oil if it weighs 610 N.
-
D Question 37 Value reinforcement means es reiterating the features included with the purchase price using the assumptive method to close a sale O getting credit for the value you create for the...
-
JDD Corporation provides the following benefits to its employee, Ahmed (age 52): Salary Health insurance Dental insurance Life insurance Dependent care Professional dues Personal use of company jet...
-
If the traffic speed of the requirements of Iason SA is 5 what is the average collection time of receivables? a. 75 b. 73 c. 78 d. none

Study smarter with the SolutionInn App


