Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HI(aq) + RbOH(aq) b.
Question:
Write balanced complete ionic and net ionic equations for each acid–base reaction.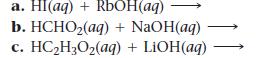
Transcribed Image Text:
a. HI(aq) + RbOH(aq) b. HCHO₂(aq) + NaOH(aq) c. HC₂H₂O₂(aq) + LiOH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
The balanced complete ionic and net ionic equations f...View the full answer

Answered By

Larlyu mosoti
I am a professional writer willing to do several tasks free from plagiarism, grammatical errors and submit them in time. I love to do academic writing and client satisfaction is my priority. I am skilled in writing formats APA, MLA, Chicago, and Harvard I am a statistics scientist and I can help out in analyzing your data. I am okay with SPSS, EVIEWS, MS excel, and STATA data analyzing tools.
Statistical techniques: I can do linear regression, time series analysis, logistic regression, and some basic statistical calculations like probability distributions. . I'm ready for your working projects!
Services I would offer:
• Academic writing.
• Article writing.
• Data entry.
• PDF conversion.
• Word conversion
• Proofreading.
• Rewriting.
• Data analyzing.
The best reason to hire me:
- Professional and Unique work in writing.
- 100% satisfaction Guaranteed
- within required time Express delivery
- My work is plagiarism Free
- Great communication
My passion is to write vibrantly with dedication. I am loyal and confident to give my support to every client. Because Client satisfaction is much more important to me than the payment amount. A healthy client-contractor relationship benefits in the longer term. Simply inbox me if you want clean work.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write complete ionic and net ionic equations for each of the following molecular equations. a. 2HNO3(aq) + Mg(OH)2(s) 2H2O(l) + Mg(NO3)2(aq) Nitric acid, HNO3, is a strong electrolyte. b....
-
Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq) HO(1)+ LiCI(aq) b. Cas(aq) + CuCl(aq) CuS(s) + CaCl(aq) c. NaOH(aq) + HCHO(aq) d. Na3PO4(aq) + NiCl(aq)...
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HBr(aq) + NaOH(aq) b. HF(aq) + NaOH(aq) C. HCHO(aq) + RbOH(aq)
-
2. National Defense (40 points). There are 11 countries in Europe who get utility from general consumption c, and from European national defense G. The utility of a generic country i is u(ci, G) =...
-
Why are competent employees important to an organizations internal control system?
-
A systems analyst must be both technically proficient and capable of successful customer communication. Developing a good system requires a complete understanding of user requirements. Many times,...
-
What simple and practical steps could Rolando take to help evaluate the effectiveness of training provided to his new employees to prepare them to work safely and be hospitable to guests?
-
The following transactions were incurred by Dimasi Industries during January 2010: 1. Issued $800,000 of direct material to production. 2. Paid 40,000 hours of direct labor at $18 per hour. 3....
-
A car has a sticker price of $69,000. The car has a 100 hp engine and can accelerate from 0 to 60 mph in 15.8 seconds. The lease rate is 4.6%. The term of the lease is three years. The buyout is...
-
Suppose that a customer at Bank of America demands that $280,000 be transferred to a competing commercial bank because the competing commercial bank is offering higher deposit rates. What difficulty...
-
A 25.00-mL sample of an unknown HClO 4 solution requires titration with 22.62 mL of 0.2000 M NaOH to reach the equivalence point. What is the concentration of the unknown HClO 4 solution? The...
-
Complete and balance each acidbase equation. a. HI(aq) + LiOH(aq) b. HCHO(aq) + Ca(OH)(aq) c. HCl (aq) + Ba(OH)(aq)
-
The following control inputs are active in the bus system shown in Fig. 5-4. For each case, specify the register transfer that will be executed during the next clock transition. Fig. 5-4 a. b. C. d....
-
The accounting records of the Eco Paper Company include the following information relating to the current year ended 31 March 2023: Materials 31 March 2023 $20,000 1 April 2022 $25,000 Work in...
-
The first read is an article on the development of money of a World War II prisoner-of-war, which was published in 1945. The second article was published in the opinion section of the New York Times...
-
Describe each Speaker's basic assumptions regarding employee motivation. That is, what are the underlying principles which guide how the Speaker treats his/her people (i.e., their direct report...
-
Find the area of the shaded region. The graph to the rate of IQ scores of adults, and those scores are normally distributed with the mean of 100 and a standard deviation of 15. x=81
-
In which scenario is Nikki showing resilience to stress? Nikki lost her job as an engineer 3 months ago. At first, she was depressed, but she realized she wanted to change career paths and decided to...
-
Is it possible to have a copper-zinc alloy that, at equilibrium, consists of an phase of composition 80 wt% Zn-20 wt% Cu, and also a liquid phase of composition 95 wt% Zn-5 wt% Cu? If so, what will...
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
An object is dropped from a height of 53 in. Neglecting air resistance, how long would it take for the body to strike the ground? Use a = g = 32.2 ft/s 2 . A body starting from rest with constant...
-
Calculate the kinetic energy in Nm of a 15-kg mass if it has a velocity of 1.20 m/s. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Calculate the kinetic energy in Nm of a 3600-kg truck moving at 16 km/h. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
which of the following stateents is consistent with the balance sheet model of a firm a. longterm investment decision b. shareholder value equals long term liabilities minus short term liabilities c....
-
please show all methods for these please pleaseee! y 4. Equity at start of year 120,000 Sales revenue 175,000 Current liabilities at end of the year 90,000 Non-current liabilities at end of the year...
-
if the returns between two assets are negatively correlated, then the standard deviation of a portfolio made up of the two assets is: A) equal to a weighted average of the individual asset's standard...

Study smarter with the SolutionInn App


