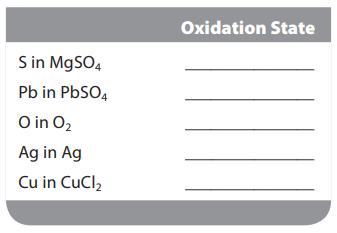
Assign the oxidation state for the element listed in each of the following compounds: S in MgSO4
Question:
Assign the oxidation state for the element listed in each of the following compounds:
Transcribed Image Text:
S in MgSO4 Pb in PbSO4 O in O₂ Ag in Ag Cu in CuCl₂ Oxidation State
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
1 S in MgSO4 Magnesium sulfate Mg is in Group 2 and typically has an oxidation state of 2 ...View the full answer

Answered By

HARSH RANJAN
Taken classes at college to graduates, Also worked as an expert to a freelancer online question-solving portal for more than 8 months with an average rating greater than 4.2 out of 5.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
n i=1 - - let denote the sample correlation coefficient between Y and X (defined formally below), and let = 1 (yi ) and = - 1(xi). (These notations are also defined in slides 44-46 of Chapter 1...
-
Indicate the oxidation state for the element noted in each ssof the following: a. H in CaH2 b. H in H2O c. C in CH4 d. S in H2SO4
-
Indicate the oxidation state for the element noted in each of the following: a. H in H2 b. H in C2H4 c. Si in SiH4 d. N in HNO3
-
In Boolean algebra, the bar sign (-) indicates: Select one: a. NOT operation b. AND operation C. OR operation d. All of the mentioned
-
In conducting interviews and observing factory operations to implement an activity-based costing system, you determine that several activities are unnecessary or redundant. For example, warehouse...
-
The article Most Customers OK with New Bulbs (USA Today, Feb. 18, 2011) describes a survey of 1,016 randomly selected adult Americans. Each person in the sample was asked if they have replaced...
-
Simon and Sherry divorce during the current year. As part of their property settlement, Simon gives Sherry 25% of the stock in his 100%-owned corporation, Hobday, Inc. The stock has a fair market...
-
For more than 75 years, New England Foundry, Inc., has manufactured wood stoves for home use. In recent years, with increasing energy prices, George Mathison, president of New England Foundry, has...
-
Sheffield Company prepares monthly cash budgets. Relevant data from operating budgets for 2022 are as follows. January February Sales $468,000 $520,000 Direct materials purchases 156,000 162,500...
-
The Leadership Challenge Case Study - Jacinta Arden-Leading New Zealand through the Covid-19 Pandemic QUESTIONS TO ADDRESS IN YOUR REPORT: 1. Examine the impacts of a global pandemic and...
-
Consider the reaction between oxygen (O 2 ) gas and magnesium metal to form magnesium oxide. Using oxidation states, how many electrons would each oxygen atom gain, and how many electrons would each...
-
Consider the reaction between sodium metal and fluorine (F 2 ) gas to form sodium fluoride. Using oxidation states, how many electrons would each sodium atom lose, and how many electrons would each...
-
1. What pressured or motivated Ducati to implement new digital technology? 2. What costs could Ducati cut? What costs could it not cut? For example, could Ducati cut its research and development...
-
You've decided to build a radio to listen to your favourite FM radio station, which broadcasts at 101.5 MHz. For the tuner, you'll be using an RLC circuit, but the only inductor you happen to have on...
-
You are the lead buyer for a large healthcare organization in British Columbia and have been tasked with leading the procurement of a new CAT Scan Machine. Outline four steps to prepare and call a...
-
A circuit is composed of a coil having N turns and area A. Its leads are connected to a combination of resistors, as shown in (Figure 1). All three resistors have the same resistance R. The coil is...
-
Let two planes be given by 2x-y+z = 8 and z = x+y-5 (a) Find the angle between the two planes. Leave your answer in degrees and round to the nearest tenth. (b) Find the vector equation of the line of...
-
9-2. The profile of a gear tooth shown in Fig. P9.2 is approximated by the trigonometric equation y(x) = a. Estimate the area A using eight rectangles of equal width A x = 1/8, b. Calculate the exact...
-
What are some of the sources of foreign material on the surface of manufactured products?
-
In a large midwestern university, 30% of the students live in apartments. If 200 students are randomly selected, find the probability that the number of them living in apartments will be between 55...
-
You isolate a compound with the formula PtCl 4 . 2KCl. From electrical conductance tests of an aqueous solution of the compound, you find that three ions per formula unit are present, and you also...
-
Which of following statement(s) is(are) true? a. Phosphoric acid is a stronger acid than nitric acid. b. The noble gas with the lowest boiling point is helium. c. Sulfur is found as the free element...
-
The atmosphere contains 9.0 10 -6 % Xe by volume at 1.0 atm and 25 C. a. Calculate the mass of Xe in a room 7.26 m by 8.80 m by 5.67 m. b. A typical person takes in about 2 L of air during a...
-
__________________________________________ I have calculated (not sure if it's correct): 2019 ROE 3.74% 2018 ROE -19.85% 2019 RONA 1.91% 2019 NOPM 7.74% 2019 NOAT 24.62% 2018 RONA -9.91% 2018 NOPM...
-
When constructing a budget, it is helpful to use a personal cash flow statement, which measures a person's _______ and _______. A. Cash inflows; cash outflows B. Assets; expenses C. Assets;...
-
Wildhorse Inc. issued $6 million of 10-year, 8% convertible bonds on June 1, 2020, at 99 plus accrued interest. The bonds were dated April 1, 2020, with interest payable April 1 and October 1. Bond...

Study smarter with the SolutionInn App


