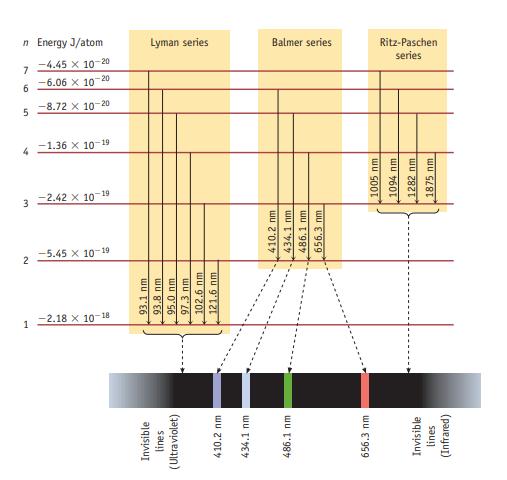
A photon with a wavelength of 93.8 nm strikes a hydrogen atom, and light is emitted by
Question:
A photon with a wavelength of 93.8 nm strikes a hydrogen atom, and light is emitted by the atom. How many emission lines would be observed? At what wavelengths? Explain briefly (see Figure 6.10).
Data given in figure 6.10
Transcribed Image Text:
Invisible lines (Ultraviolet) 410.2 nm 434.1 mm 486.1 mm 656.3 mm Invisible lines (Infrared) 1 -2.18 X 10-18 93.1 nm 93.8 nm 95.0 nm 97.3 nm 102.6 nm 121.6 nm N -5.45 x 10-19 3 -2.42 x 10-19 410.2 mm 434.1 nm. 486,1 nm 656.3 nm 1005 nm 1094 nm 1282 nm 1875 nm 7 -1.36 x 10-19 5 -8.72 x 10-20 -6.06 x 10-20 -4.45 x 10-20 n Energy J/atom 9 7 Lyman series Balmer series series Ritz-Paschen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The number of emission lines that would be observed depends on the energy levels of the hydrogen ato...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Canadian Steel: Role of Information Systems (IS) Canadian Steel is the 5th largest integrated steel manufacturer in North America and the largest in Canada. Headquartered in Mitchell, Ontario, it can...
-
Consider only transitions involving the n = 1 through n = 4 energy levels for the hydrogen atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the four quantum...
-
Consider only transitions involving the n = 1 through n = 5 energy levels for the H atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the five quantum...
-
Read the Poem Little Birds Flying and answer the following questions: What it is notifying? To whom it is notifying? How is the work two dimensional? What does it take to realize the project? ...
-
Refer to Problem M4-8. There is a saddle point in this game, making it a pure strategy game. Ignore this and solve it as a mixed strategy game. What special condition in the solution indicates that...
-
Given the network in figure, find the power supplied and the average power absorbed by eachelement. $20 320 6/0A t VT j2n -j1a
-
What is the difference between make-to-order and assemble-to-order? Give an example of each.
-
At a university faculty meeting in 2000, a proposal was made to increase the housing benefits for new faculty to keep pace with the high cost of housing. What will likely be the long-run effect of...
-
Revise your worksheet to reflect these updated assumptions and then answer the questions that follow. Required: . Use your spreadsheet to recalculate the amounts related to the stock transactions and...
-
In principle, which of the following can be determined? (a) The energy of an electron in the H atom with high precision and accuracy (b) The position of a high-speed electron with high precision and...
-
Which of these are observable? (a) Position of an electron in an H atom (b) Frequency of radiation emitted by H atoms (c) Path of an electron in an H atom (d) Wave motion of electrons (e) Diffraction...
-
Explain the difference between gross earnings and net earnings for a payroll period.
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Give a brief general description of the number of degrees of freedom. A. The number of degrees of freedom for a collection of sample data is the number of unique, non-repeated sample values. The...
-
Suppose you are given a data frame df. df = pd.DataFrame({'Click_ID':['A', 'B', 'C', 'D'], 'Count':[100, 200, 300, 400]}) In many data science projects, you are required to convert a dataframe into a...
-
Refer to the table on the top of the next column. If the price of a fudge bar is $2, the price of a Popsicle is $1, and a student has $9 to spend, what quantities will she purchase at a consumer...
-
Determine the values of the given trigonometric functions directly on a calculator. The angles are approximate. tan 0.8035
-
Why is it not necessary to know absolute half-cell potentials to determine the emf of an electrochemical cell?
-
What is the voltage between the terminals of a battery in which the contents are in chemical equilibrium?
-
By convention, the anode of a battery is where oxidation takes place. Is this true when the battery is charged, discharged, or both?
-
Elite Lawn & Plowing (EL&P) is a lawn and snow plowing service with both residential and commercial clients. The owner believes that the commercial sector has more growth opportunities and is...
-
Rowan Company is considering two alternative investment projects. Each requires a $263,000 initial investment. Project A is expected to generate net cash flows of $73,000 per year over the next six...
-
A company purchased 300 units for $30 each on January 31. It purchased 390 units for $39 each on February 28. It sold a total of 440 units for $40 each from March 1 through December 31. What is the...

Study smarter with the SolutionInn App


