A potential of 0.142 V is recorded (under standard conditions) for a voltaic cell constructed using the
Question:
A potential of 0.142 V is recorded (under standard conditions) for a voltaic cell constructed using the following half reactions: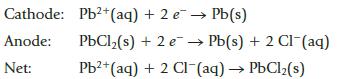
(a) What is the standard reduction potential for the anode reaction?
(b) Calculate the solubility product, Ksp, for PbCl2.
Transcribed Image Text:
Cathode: Anode: Net: Pb2+ (aq) + 2 e PbCl₂(s) + 2 e. Pb²+ (aq) + 2 Cl(aq) → PbCl₂(s) Pb(s) Pb(s) + 2 Cl- (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a To find the standard reduction potential for the anode reaction we can use the following equation ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A cell is constructed using the following half-reactions: (a) What reactions should be observed at the anode and cathode? (b) Calculate the solubility product constant, K sp , for Ag 2 SO 4 . Ag+...
-
Under standard conditions for all concentrations, the following reaction is spontaneous at 25oC. O2(g) + 4H + (aq) + 4Br(aq) 2H2O(l) + 2Br2(l) If [H+] is decreased so that the pH = 3.60, what value...
-
Under standard conditions for all concentrations, the following reaction is spontaneous at 25oC. O3(g) + 2H + (aq) + 2Co2+(aq) O2(g) + H2O(l) + 2Co3+(l) If [H+] is decreased so that the pH = 9.10,...
-
Svengool Inc. financial statements included the following amounts for the current year: Retired bonds $67,000 30,000 Proceeds from collection of note receivable Dividends received 11,000 Acquired...
-
Borders and Amazon.com are competitors in vending books and other consumer items. The two are differentiated to an extent by their marketing strategies. Although Amazon.com relies exclusively on...
-
During the last year, you have had a loan commitment from your bank to fund inventory purchases for your small business. The total line available was $500,000, of which you took down $400,000. It is...
-
What steps would you suggest that the resorts owners take to prevent being victimized by potential invoice frauds of this type?
-
Slow Ride Corp. is evaluating a project with the following cash flows: Year Cash Flow 0....$29,000 1....11,200 2....13,900 3....15,800 4....12,900 5....-9,400 The company uses a 10 percent interest...
-
Figaro Production bought a machine at the beginning of the year at a cost of $22,350. The estimated useful life was five years and the residual value was $2,000. Required: 1. Complete a depreciation...
-
Which of the following reactions is (are) product-favored at equilibrium? (a) Zn(s) + 1(s) Zn+ (aq) + 2 I (aq) (b) 2 Cl (aq) + (s) Cl(g) + 2 [ (aq) (c) 2 Nat (aq) + 2 Cl (aq) 2 Na(s) + Cl(g) (d) 2...
-
You want to set up a series of voltaic cells with specific cell potentials. A Zn 2+ (aq, 1.0 M) | Zn(s) half-cell is in one compartment. Identify several half-cells that you could use so that the...
-
Atlantic Surf manufactures surfboards. The companys sales budget for the next three months is shown below. In addition, company policy is to maintain finished goods inventory equal (in units) to 40%...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
For the linear systems in Exercise 3, verify that the error vector Ax - b resulting from the least squares solution x is orthogonal to the column space of A. (a) (b) 707 112 6093 1211
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
(a) Implement the function F 1 = A'BC + B'C + ABC using an FPGA with programmable logic blocks consisting of 4-to-1 multiplexers. Assume inputs and their complements are available. (b) Implement the...
-
(a) In which applications should a designer use a CPLD rather than an FPGA? (b) In which applications should a designer use an MPGA rather than an FPGA? (c) In which applications should a designer...
-
(a) What is the difference between a traditional gate array and an FPGA? (b) What are the different types of FPGAs based on architecture (organization)? (c) What are the different programming...
-
Adirondack Marketing Inc. manufactures two products, A and & Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products, However, management is...
-
9. value: 10.00 points < Question 9 (of 10) P3-18 Using the Du Pont Identity [LO3] Y3K, Inc., has sales of $4,500, total assets of $3,460, and a debt-equity ratio of 1.40. If its return on equity is...
-
Consider the simple income statement model below. The company would like donate 15% of its net income to charity. How much will they contribute? Contribution Rate 15% Income Tax Rate 40% Circular...

Study smarter with the SolutionInn App


