Which of the following reactions is (are) product-favored at equilibrium? (a) Zn(s) + 1(s) Zn+ (aq)
Question:
Which of the following reactions is (are) product-favored at equilibrium?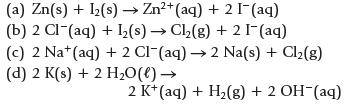
Transcribed Image Text:
(a) Zn(s) + 1₂(s) → Zn²+ (aq) + 2 I¯ (aq) (b) 2 Cl (aq) + ₂(s)→ Cl₂(g) + 2 [¯ (aq) (c) 2 Nat (aq) + 2 Cl (aq) →2 Na(s) + Cl₂(g) (d) 2 K(s) + 2 H₂O(l) → 2 K+ (aq) + H₂(g) + 2 OH-(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The standard Gibbs free energy change can be calculated using the following equation Gf Gfproducts G...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions will have the largest equilibrium constant (K) at 298 K? a) Fe2O3(s) + 3 CO(g) ? 2 Fe(s) + 3 CO2(g) ?G = -28.0 kJ b) It is not possible to determine without more...
-
1) Express the equilibrium constant for the following reaction. P 4 O 10 (s) ? P 4 (s) + 5 O 2 (g) a) K = [O 2 ] -5 b) K = [O 2 ] 5 c) K = d) K = 2) For the reaction of carbon with carbon dioxide to...
-
Write a detailed paper on Trademark Law of the People's Republic of China
-
Use hand calculations to fit the multiple linear regression model 1 y = β0 + β1x1 + β2x2 to the data set in DS 13.6.2. (a) Write down the vector of observed values...
-
Employees expect that all parties will honor their explicit and implicit obligations. Distrust occurs when these obligations are not met or when the parties have different expectations regarding the...
-
Describe the various sources of capital funding available to public firms.
-
Will the organizational structure selected by the partners have an impact on your decision to extend the loan?
-
(a) Sum up the present values of the Hernandezes assets, excluding their personal residence, and identify which assets derive from tax-sheltered accounts. (b) Assume that the Hernandezes sold their...
-
\ table [ [ \ table [ [ Falcons Corporation ( an 5 Corporation ] , [ Income Statement ] , [ December 3 1 , Year 1 and Year 2 ] ] , , ] , [ , Year 1 , Year 2 ] , [ Sales revenue,$ 3 0 0 , 0 0 0 , $ 4...
-
You want to set up a series of voltaic cells with specific cell potentials. The Ag + (aq, 1.0 M) | Ag(s) half-cell is one of the compartments. Identify several half-cells that you could use so that...
-
A potential of 0.142 V is recorded (under standard conditions) for a voltaic cell constructed using the following half reactions: (a) What is the standard reduction potential for the anode reaction?...
-
(a) If g(x) = (sin2x)/x2, use your calculator or computer to make a table of approximate values of t g(x) dx for t = 2, 5, 10, 100, 1000, and 10,000. Does it appear that g(x) is convergent? (b) Use...
-
Loma Company manufactures basketball backboards. The following information pertains to the company's normal operations per month: Output units15,000 boards Machine-hours4,000 hours Direct...
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Let A be an m n matrix with linearly independent row vectors. Find a standard matrix for the orthogonal projection of Rn onto the row space of A.
-
Jax Incorporated reports the following data for its only product. The company had no beginning finished goods inventory and it uses absorption costing. $ 57.30 per unit $ 10.30 per unit $ 7.80 per...
-
(a) If gate delays are 5ns, what is the delay of the fastest 4-bit ripple carry adder? (b) If gate delays are 5ns, what is the delay of the fastest 4-bit adder? What kind of adder will it be?
-
Design the correction circuit for a BCD adder that computes Zdigit 0 and C for S 0 . This correction circuit adds 0110 to S 0 if S 0 > 9. This is the same as adding 0AA0 to S 0 , where A = 1 if S 0 >...
-
Consider the following programmable I/O block: Highlight the connections to configure this I/O block as an INPUT pin. Specify the five configuration bits. CONFIGURATION BITS Vcc OUT 3-STATE LATCHED...
-
View Policies Current Attempt in Progress Botticelli Inc. was organized in late 2018 to manufacture and sell hosiery. At the end of its fourth year of operation, the company has been fairly...
-
Facts Taxpayers' Data: Names Address Carl C. and Carla D. Cox 391 Virginal Place Richmond, Virginia 23224 111-01-1111 111-02-1111 SS Numbers: Carl Carla Dates of Birth: Carl Carla Marital Status...
-
downward slipping yield curves occur Question 25 The relationship between interest rates and the time to maturity is called the term structure of interest rates. liquidity effect International Fisher...

Study smarter with the SolutionInn App


