A cell is constructed using the following half-reactions: (a) What reactions should be observed at the anode
Question:
A cell is constructed using the following half-reactions: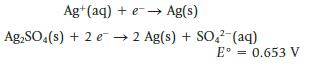
(a) What reactions should be observed at the anode and cathode?
(b) Calculate the solubility product constant, Ksp, for Ag2SO4.
Transcribed Image Text:
Ag+ (aq) + e → Ag(s) Ag₂SO4(s) + 2 e 2 Ag(s) + SO4²- (aq) E° = 0.653 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The halfreaction with the more positive standard potential will occur at the cathode Therefore the ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A voltaic cell is constructed using the reaction (a) Write equations for the oxidation and reduction half-reactions. (b) Which half-reaction occurs in the anode compartment, and which occurs in the...
-
A potential of 0.142 V is recorded (under standard conditions) for a voltaic cell constructed using the following half reactions: (a) What is the standard reduction potential for the anode reaction?...
-
A voltaic cell is constructed using the reaction of chromium metal and iron(II) ions. Complete the following sentences: Electrons in the external circuit flow from the _______ electrode to the...
-
The accounting records of Shinault Inc. show the following data for 2017 (its first year of operations). 1. Life insurance expense on officers was $9,000. 2. Equipment was acquired in early January...
-
Dayna Moore, CEO of Layton Transmissions, sat dejected in her chair after reviewing the 2011 first-quarter financial reports on one of the companys core products: a standard, five-speed transmission...
-
Explain how a countrys import trade limitations and tariffs influence MNCs foreign direct investment.
-
Assume that Mr. Bell did in fact ordinarily purchase pool chemicals for the resort. Is the resort then responsible for paying the invoice?
-
Best Parking, near an airport, incurred the following costs to acquire land, make land improvements, and construct and furnish a small building: Best Parking depreciates land improvements over 25...
-
Following are account balances (in millions of dollars) from a recent FedEx annual report, followed by several typical transactions. Assume that the following are account balances on May 31 (end of...
-
Which of the following reactions is (are) product-favored at equilibrium? (a) Zn(s) + 1(s) Zn+ (aq) + 2 I (aq) (b) 2 Cl (aq) + (s) Cl(g) + 2 [ (aq) (c) 2 Nat (aq) + 2 Cl (aq) 2 Na(s) + Cl(g) (d) 2...
-
You want to set up a series of voltaic cells with specific cell potentials. A Zn 2+ (aq, 1.0 M) | Zn(s) half-cell is in one compartment. Identify several half-cells that you could use so that the...
-
Suppose that X1, . . . , Xn form a random sample from a distribution for which the p.d.f. or the p.f. is f (x|), where the value of the parameter is unknown. Let X = (X1, . . . , Xn), and let T be a...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
Solve xy' - 2y = x.
-
Vanishing Games Corporation (VGC) operates a massively multiplayer online game, charging players a monthly subscription of $15. At the start of January 2021, VGC's income statement accounts had zero...
-
Financial data, medical service data, and staffing data are best defined as what type of data
-
. What is meant by the term "foreign exchange rate"? In general, what causes foreign exchange rates to vary over time? . Do changes in foreign exchange rates benefit or hurt U.S. companies that are...
-
Let W be the line with parametric equations x = 2t, y = - t, z = 4t (- < t < ) (a) Find a basis for W. (b) Use Formula 6 to find the standard matrix for the orthogonal projection onto W. (c) Use the...
-
Fahrad Inc. sells all of its product on account. Fahrad has the following accounts receivable payment experience: Percent paid in the month of sale .........10 Percent paid in the month after the...
-
A Mealy sequential circuit with four output variables is realized using a 22V10. What is the maximum number of input variables it can have? What is the maximum number of states? Can any Mealy circuit...
-
Show how the left shift register of Figure 2-41 could be implemented using a CPLD. Draw a diagram. Give the equations for the flip-flop D inputs. always @ (posedge CLK) begin if (CLR) else if (Ld)...
-
An N-bit bidirectional shift register has N parallel data inputs, N outputs, a left serial input (LSI), a right serial input (RSI), a clock input, and the following control signals: Load: Load the...
-
The Swift Meal has two restaurants that are open 24 hours a day. Fixed costs for the two restaurants together total $405,000 per year. Service varies from a cup of coffee to full meals. The average...
-
help 5 Instructions Label and Amount Description Retained Earnings Statement Instructions The assets and studies of Thompson Computer Services at March at the end of the current year, and its revenue...
-
All the information has been given. slides Question 1. See the numerical example from the lecture slides (Lecture 8 Slides # 39-40). Suppose the two countries were to integrate their automobile...

Study smarter with the SolutionInn App


