A weighed sample of iron (Fe) is added to liquid bromine (Br 2 ) and allowed to
Question:
A weighed sample of iron (Fe) is added to liquid bromine (Br2) and allowed to react completely. The reaction produces a single product, which can be isolated and weighed. The experiment was repeated a number of times with different masses of iron but with the same mass of bromine (see graph below).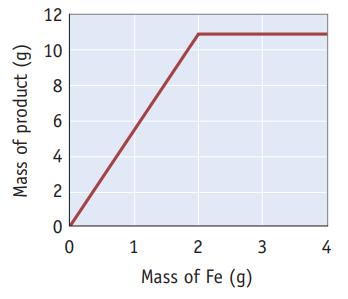
(a) What mass of Br2 is used when the reaction consumes 2.0 g of Fe?
(b) What is the mole ratio of Br2 to Fe in the reaction?
(c) What is the empirical formula of the product?
(d) Write the balanced chemical equation for the reaction of iron and bromine.
(e) What is the name of the reaction product?
(f) Which statement or statements best describe the experiments summarized by the graph?
(i) When 1.00 g of Fe is added to the Br2, Fe is the limiting reagent.
(ii) When 3.50 g of Fe is added to the Br2, there is an excess of Br2.
(iii) When 2.50 g of Fe is added to the Br2, both reactants are used up completely.
(iv) When 2.00 g of Fe is added to the Br2, 10.8 g of product is formed. The percent yield must therefore be 20.0%.
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





