Acetic acid, CH 3 CO 2 H, is made industrially by the reaction of methanol and carbon
Question:
Acetic acid, CH3CO2H, is made industrially by the reaction of methanol and carbon monoxide.
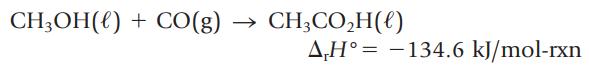
What is the enthalpy change for producing 1.00 L of acetic acid (d = 1.044 g/mL) by this reaction?
Transcribed Image Text:
CH₂OH() + CO(g) CH3CO₂H() AH° -134.6 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
enthalpy change for producing 100 L of acetic acid CH3CO2H by the given reaction you need to use the ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Ammonia gas is made industrially by the Haber process, which involves the reaction between the gases nitrogen and hydrogen. The amount of ammonia gas produced from this reaction is affected by both...
-
Tony acquired 1,000 shares in X Co (a resident public company) for $10 each in August 2000. In January this year X Co returned $7 of capital to its shareholder in respect to each share they held. The...
-
Using the information in Problem 1, determine the sample size needed if the standard time estimate is to be within 5 percent of the true mean 99 percent of the time.
-
A long constantan wire of 1-mm diameter is butt welded to the surface of a large copper block, forming a thermo-couple junction. The wire behaves as a fin, permitting heat to flow from the surface,...
-
The median sales prices for new single-family houses for the years 20042007 are as follows (Census Bureau website, March 19, 2009). a. Use 2004 as the base year and develop a price index for new...
-
The following client-prepared bank reconciliation is being examined by Kautz CPA, during an examination of the financial statements of Concrete Products, Inc. Required Identify one or more audit...
-
may be involved in some testing of calculations. Multiple Choice o Managers o Regulators o Specialists o The press o Employees
-
Assume you mix 100.0 mL of 0.200 M CsOH with 50.0 mL of 0.400 M HCl in a coffee-cup calorimeter. The following reaction occurs: The temperature of both solutions before mixing was 22.50C, and it...
-
Isooctane (2,2,4-trimethylpentane), one of the many hydrocarbons that make up gasoline, burns in air to give water and carbon dioxide. What is the enthalpy change if you burn 1.00 L of isooctane (d =...
-
Aggressive Corporation approaches Matt Taylor, a loan officer for Oklahoma State Bank, seeking to increase the companys borrowings with the bank from $100,000 to $200,000. Matt has an uneasy feeling...
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
Benicio wants to make sure that the Sales table does not contain any duplicate records, which would make any sales analysis incorrect. Identify and remove duplicate records in the Sales table as...
-
University Car Wash purchased new soap dispensing equipment that cost $261,000 including installation. The company estimates that the equipment will have a residual value of $27,000. University Car...
-
Return to the situation in Short Exercise S3-7. Here you are accounting for the same transactions on the books of Texas First Bank, which lent the money to Mizuno Travel. Perform all three steps of...
-
If |62x|>9, which of the following is a possible value of x? A. 2 B. 1 C. 0 D. 4 E. 7
-
Count the total number of s bonds and p bonds in the compound below: -3-N
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Use the tabulated values of the enthalpy of combustion of benzene and the enthalpies of formation of CO 2 (g) and H 2 O(l) to determine H o f for benzene.
-
The following information is available for a company's maintenance cost over the last seven months. Month June July August September October November December Units Produced 90 180 120 150 210 240 60...
-
2019 Tax Rate Schedules Individuals Schedule X-Single If taxable income is over: But not over: The tax is: $ 0 $ 9,700 10% of taxable income $ 9,700 $ 39,475 $970 plus 12% of the excess over $9,700 $...
-
Question 8 [5 points) Choose the term that best matches each of the following descriptions: select answer A form of partnership that is permitted for professionals such as lawyers and accountants,...

Study smarter with the SolutionInn App


