Answer the following questions based on Figure 6.2: (a) Which type of radiation involves less energy, x-rays
Question:
Answer the following questions based on Figure 6.2:
(a) Which type of radiation involves less energy, x-rays or microwaves?
(b) Which radiation has the higher frequency, radar or red light?
(c) Which radiation has the longer wavelength, ultraviolet or infrared light?
Data given in figure 6.2
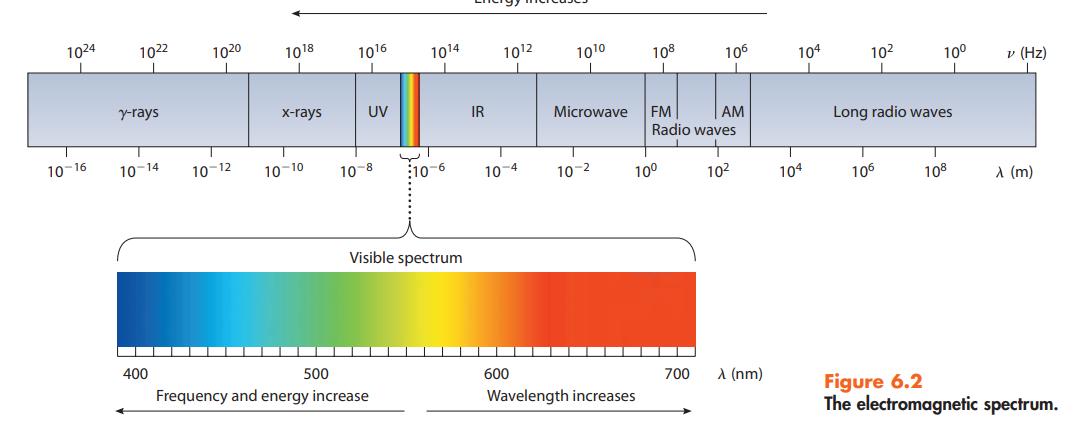
Transcribed Image Text:
1024 10-16 1022 y-rays 10-14 400 1020 10-12 1018 x-rays 10-10 1016 UV 10-8 ان 500 Frequency and energy increase 1014 10-6 Visible spectrum IR 10¹2 10-4 ▬ 600 10¹⁰ Microwave 10-2 108 Wavelength increases 10⁰ FM AM Radio waves 106 700 10² λ (nm) 104 I 104 10² 10⁰ Long radio waves 106 108 v (Hz) A (m) Figure 6.2 The electromagnetic spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a In the electromagnetic spectrum as shown in Figure 62 xrays are located to the left of m...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Answer the following questions based on the information presented for Cloud 9 in Appendix B of this book and in the current and earlier chapters. You should also consider your answers to the case...
-
Answer the following questions based on two assumptions: (1) Inflation increases the prices of all goods by 20%. (2) Ina's income increases from $50,000 to $55,000. a. Has Ina's budget line become...
-
Answer the following questions based on the information presented for Cloud 9 in Appendix B and in the current and earlier chapters. You should also consider your answers to the case study questions...
-
Fill the blanks of the following table (last column) and find out the value of SDR in terms of U.S. dollars ($/SDR) and the value of U.S. dollars in terms of SDR (SDR/$) The exchange rate is...
-
Solve the following LP problem first graphically and then by the simplex algorithm: Maximize profit = 4X1 + 5X2 Subject to X1 + 2X2 80 3X1 + X2 75 X1, X2 0 What are the values of the basic...
-
Stock Valuation a substantial percentage of the companies listed on the NYSE and NASDAQ dont pay dividends, but investors are nonetheless willing to buy shares in them. How is this possible given...
-
What are the advantages and disadvantages of building permanent venues v. the utilisation of temporary venues for a multi-sports event?
-
Consider the unadjusted trial balance of Kings, Inc., at August 31, 2010, and the related month-end adjustment data. Adjustment data at August 31, 2010 include the following: a. Accrued advertising...
-
"YURVITTUVUI. (The following information applies to the questions displayed below.) The following is the post-closing trial balance for the Whitlow Manufacturing Corporation as of December 31, 2020....
-
Sublimation of 1.0 g of dry ice, CO 2 (s), forms 0.36 L of CO 2 (g) (at 78C and 1.01 10 5 Pa). The expanding gas can do work on the surroundings. Calculate the amount of work done on the...
-
In the lab, you plan to carry out a calorimetry experiment to determine r H for the exothermic reaction of Ca(OH) 2 (s) and HCl(aq). Predict how each of the following will affect the calculated...
-
Problems 6675. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for later sections, a final exam, or subsequent courses such as calculus. For f...
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
What is the relationship between the gross public debt and the net public debt?
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
Lactones can be prepared from diethyl malonate and epoxides. Diethyl malonate is treated with a base, followed by an epoxide, followed by heating in aqueous acid: Using this process, identify what...
-
Predict the major product of the following transformation. CO2ET C10H100 Heat
-
Consider the structures of the constitutional isomers, Compound A and Compound B (below). When treated with aqueous acid, Compound A undergoes isomerization to give a cis stereoisomer. In contrast,...
-
Swamp Ltd acquired 90% of the shares of Tortoise Ltd on 1 July 2015 for $237 000. At this date, the equity of Tortoise Ltd consisted of: Share capital Asset revaluation surplus Retained earnings 125...
-
1. Which of the following statements about integrated accounting software is true? Mid-range (or mid-level) accounting software would not be ideal for a multinational company because typical programs...
-
Sunrise Coffee Shop, in an effort to streamline its accounting system, has decided to utilize a cash receipts journal. Record the following transactions for the first two weeks in March, total the...

Study smarter with the SolutionInn App


