Balance the following equations, and then classify each as a precipitation, acidbase, or gas-forming reaction. (a) Ba(OH)(aq)
Question:
Balance the following equations, and then classify each as a precipitation, acid–base, or gas-forming reaction.
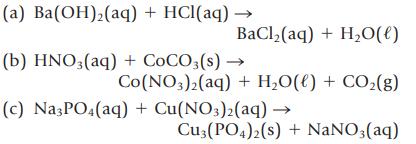
Transcribed Image Text:
(a) Ba(OH)₂(aq) + HCl(aq) → BaCl,(aq) + H,O(l) (b) HNO3(aq) + COCO3(s) → Co(NO3)2(aq) + H₂O(l) + CO₂(g) (c) Na3PO4(aq) + Cu(NO3)2(aq) → Cu3(PO4)2(s) + NaNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To balance and classify each of the given chemical reactions well first balance the equations and ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations, and then classify each as a precipitation, acidbase, or gas-forming reaction. (a) KCO3(aq) + Cu(NO3)2(aq) CuCO3(s) + KNO3(aq) (b) Pb(NO3)2(aq) + HCl(aq) PbCl(s) +...
-
Balance equations for these reactions that occur in aqueous solution, and then classify each as a precipitation, acidbase, or gas-forming reaction. Show states for the products (s, , g, aq), give...
-
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C3H6(g) + O2 (g) CO2 (g) + H2O(g) (b) NH4NO3(s) N2O(g) + H2O(g) (c) C5H6O(I) +...
-
Suppose that the following equations describe an economy: C = 170 + 0.60YD M s = 735; P = 1 T = 200 I = 100 - 4i Md = 0.75Y - 6i G = 350 a) what is the equation for equilibrium in the goods market?...
-
Suppose there is a reduction in aggregate real money demand, that is, a negative shift in the aggregate real money demand function. Trace the short-run and long-run effects on the exchange rate,...
-
N sources of current with different emf's are connected as shown in Fig. 3.40. The emf's of the sources are proportional to their internal resistances, i.e. ε = aR, where a is an assigned...
-
CAPITAL BUDGETING CRITERIA A company has a 12% WACC and is considering two mutually exclusive investments (that cannot be repeated) with the following cash flows: 0 2 3 2$180 $0 $850 $134 2$100 $134...
-
James Kirk is a financial executive with McDowell Enterprises. Although James Kirk has not had any formal training in finance or accounting, he has a good sense for numbers and has helped the company...
-
Current Year Prior Year $ 167,000 87,500 605,500 860,000 343,000 (159,500) $1,043,500 $ 110,300 74,000 529,000 713,300 302,000 (105,500) $ 909,800 Assets Cash Accounts receivable Inventory Total...
-
Which two of the following reactions are oxidation reduction reactions? Explain your answer briefly. Classify the remaining reaction. (a) CdCl(aq) + NaS(aq) CdS(s) + 2 NaCl(aq) (b) 2 Ca(s) + O(g) 2...
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. 2+ (a) CrO (aq) + 3 Sn+ (aq) + 14 H3O+ (aq) 2 Cr+ (aq) + 3...
-
Compute ending merchandise inventory and cost of goods sold for Cambridge using the weighted-average inventory costing method. The periodic inventory records of Cambridge Prosthetics indicate the...
-
A common trade off related to financial services include a. minimum deposit vs. maximum deposit b. availability vs. liquidity c. liquidity vs. access to funds d. convenience vs. fees e. RRSP vs RESP
-
Question 2. You won a free ticket to a Bruce Springsteen concert (next Friday) in a lottery. However, Rihanna is singing on the same evening. A ticket to the Rihanna concert is priced at $100 though...
-
Assignment 3 This assignment is based on content discussed in modules 3 - 5 and test basic concepts of statistical inference theory and probability distributions. Learning outcomes Work on problems...
-
Machine cost = $15,000; life = 8 years; salvage value = $3,000. What minimum cash return would an investor demand annually from the operation of this machine if he desires interest annually at the...
-
Write a program that prompts for the student's name, the number of exams, the exam score of each exam, and display the letter grade for the student. Read the entire problem description before coding....
-
Transactions made by Mickelson Co. for the month of March are shown below. Prepare a tabular analysis that shows the effects of these transactions on the expanded accounting equation, similar to that...
-
How is use of the word consistent helpful in fraud reports?
-
Starting with cyclohexene and using any other reagents of your choice, show how you would prepare each of the following compounds. a. b. c. OH OMe
-
When 1, 4-dioxane is heated in the presence of HI, compound A is obtained: a. Draw the structure of compound A. b. If one mole of dioxane is used, how many moles of compound A are formed? c. Show a...
-
Tetrahydrofuran (THF) can be formed by treating 1, 4-butanediol with sulfuric acid. Propose a mechanism for this transformation. H,SO, H;SO. 1,4-Butanediol Tetrahydrofuran (THF)
-
The Lenzie Corporation's common stock has a beta of 1.15. If the risk free rate is 3.5% and the expected retum on the market is what is the company's cost of equity capital? (Do hot round...
-
PnR Catering Ltd.is a limited company (the Company) which engages in the airline catering services and owns a multi-storey building next to the Hong Kong International Airport, where all the food...
-
Matrix Co.'s beginning and ending inventories for the month of October are: BEGINNING ENDING DIRECT MATERIALS 67,000.00 60,000.00 WORK IN PROCESS 145,000.00 170,000.00 FINISHED GOODS 85,000.00...

Study smarter with the SolutionInn App


