In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent
Question:
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent.
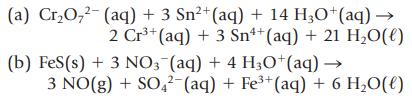
Transcribed Image Text:
2+ (a) Cr₂O₂² (aq) + 3 Sn²+ (aq) + 14 H3O+ (aq) → 2 Cr³+ (aq) + 3 Sn+ (aq) + 21 H₂O(l) (b) FeS (s) + 3 NO3(aq) + 4 H3O+ (aq) → 3 NO(g) + SO4² (aq) + Fe³+ (aq) + 6 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine which reactant is oxidized and which is reduced in each of the given reactions you can ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. (a) CH4(g) + 3 O(g) 2 CO(g) + 2 HO(l) (b) Si(s) + 2 Cl(g) ...
-
Using real employed samples, multiple field studies indicate that fat women experience more negative outcomes than fat men. In one study conducted by Steven Gortmaker and colleagues and using over...
-
Multinationals generally have production plants in a number of countries. Consequently, they can move production from expensive locations to cheaper ones in response to various economic developmentsa...
-
Find the magnetic induction at the centre of a rectangular wire frame whose diagonal is equal to d = 16 cm and the angle between the diagonals is equal to = 30; the current flowing in the frame...
-
NPV PROFILES: SCALE DIFFERENCES A company is considering two mutually exclusive expansion plans. Plan A requires a $40 million expenditure on a large-scale integrated plant that would provide...
-
Label the following transactions: a. A Nevada Corporation formed a corporation in Florida and transferred all assets to it for 100 percent of its stock. It then distributed the stock to its...
-
On June 1, Lily Company borrows $90,000 from First Bank on a 6-month, $90,000, 8% note. Prepare the entry on June 1. (Credit account titles are automatically indented when amount is entered. Do not...
-
Balance the following equations, and then classify each as a precipitation, acidbase, or gas-forming reaction. (a) Ba(OH)(aq) + HCl(aq) BaCl,(aq) + H,O(l) (b) HNO3(aq) + COCO3(s) Co(NO3)2(aq) +...
-
For each reaction, write an overall, balanced equation and the net ionic equation. (a) The reaction of aqueous lead(II) nitrate and aqueous potassium hydroxide (b) The reaction of aqueous copper(II)...
-
Describe the features of a TFSA. How do you determine the contribution limit for a TFSA? What is the purpose of the various TFSA account types? What investments qualify to be held inside a TFSA...
-
Considering only the vertical stabilizer and rudder, explain the aerodynamic forces and moments that are created. You must include at least applicable airfoil terminology, description of force...
-
part. Review A bicycle wheel is rotating at 47 rpm when the cyclist begins to pedal harder, giving the wheel a constant angular acceleration of 0.44 rad/s. Part B How many revolutions does the wheel...
-
Suppose the number of students who register for a certain class each semester can be modeled by a Poisson distribution with average 10. Suppose further that each student passes the class with...
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
Tilton Corporation has the following transactions during August of the current year. Indicate (a) The basic analysis (b) The debit-credit analysis illustrated on pages 111-116. Aug. 1 Issues shares...
-
According to a New York Times columnist, The estate tax affects a surprisingly small number of people. In 2003, . . . just 1.25 percent of all deaths resulted in taxable estates, with most of them...
-
When ethylene glycol is treated with sulfuric acid, 1, 4-dioxane is obtained. Propose a mechanism for this transformation: H,SO, Ethylene glycol 1,4-Dioxane
-
The Williamson ether synthesis cannot be used to prepare tert-butyl phenyl ether. a. Explain why this method cannot be used in this case. b. Suggest an alternative method for preparing tert-butyl...
-
Methylmagnesium bromide reacts rapidly with ethylene oxide, it reacts slowly with oxetane, and it does not react at all with tetrahydrofuran. Explain this difference in reactivity. Oxetane Ethylene...
-
Please prepare a balance sheet (comparative & classified in proper format) for fiscal year 2015 and 2016 B G H N 3 4 LO 66,200 List of Items for Statement of Cash Flows Statement 3 December 31, 2016...
-
Barlow Company manufactures three productsA, B, and C. The selling price, variable costs, and contribution margin for one unit of each product follow: Product A B C Selling price$180 $270 $240...
-
please fill out with excel formula 2 The Munchkin Theater Planning Budget For the Year Ended December 31 3 4 Planning Budget 5 5 6 Number of productions (9) 7 Number of performances(92) 8 60 Cost...

Study smarter with the SolutionInn App


