For the reaction TiCl 2 (s) + Cl 2 (g) TiCl 4 (), r G
Question:
For the reaction TiCl2(s) + Cl2(g) → TiCl4(ℓ),
ΔrG° = −272.8 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of Δf G° for TiCl2(s).
Data given in Appendix L
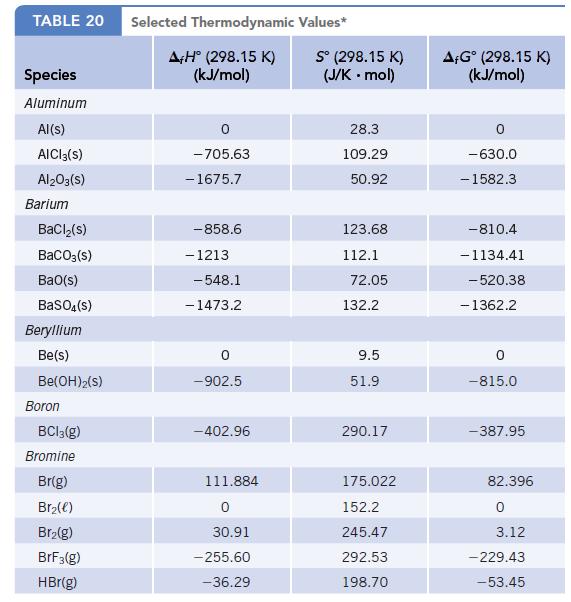
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+H° (298.15 K) (kJ/mol) -705.63 - 1675.7 -858.6 - 1213 -548.1 -1473.2 0 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K.mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 AFGᵒ (298.15 K) (kJ/mol) -630.0 - 1582.3 -810.4 -1134.41 -520.38 - 1362.2 0 -815.0 -387.95 82.396 0 3.12 -229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the standard Gibbs free energy change for the formation of TiCl2s from its elements at ...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For the reaction BaCO 3 (s) BaO(s) + CO 2 (g), r G = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of f G for BaCO 3 (s). Data given in Appendix L...
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The Regal Cycle Company manufactures three types of bicycles-a dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Sales Variable manufacturing and...
-
What advice would you give to Michelle about her selection of participants for her focus groups? Michelle had been a student union welfare officer on a years sabbatical from her undergraduate...
-
Describe how a global project can be more complex than a project performed within just one country. How might these elements affect the successful outcome of the global project?
-
. Consider the data in Figure 3. Do you agree that there appears to be no Significant difference between passengers in the front and the rear of the airplane? Why or why not? There does seem to be a...
-
The production department in a process manufacturing system completed 191,500 units of product and transferred them to finished goods during a recent period. Of these units, 31,500 were in process at...
-
Exercise 2 3 - 3 ( Algo ) Sell or process LO P 2 Cobe Company has manufactured 2 6 5 partially finished cabinets at a cost of $ 6 6 , 2 5 0 . These can be sold as is for $ 7 9 , 5 0 0 . Instead, the...
-
Determine whether the reactions listed below are entropy-favored or disfavored under standard conditions. Predict how an increase in temperature will affect the value of r G. (a) N 2 (g) + 2 O 2 (g)...
-
Using values of f H and S, calculate the standard molar free energy of formation, f G, for each of the following: (a) Ca(OH) 2 (s) (b) Cl(g) (c) Na 2 CO 3 (s) Compare your calculated values of f G...
-
You have a large supply of very rusty $2 \mathrm{in}$. sch 40 steel pipe, which you want to use for a pipeline. Because rusty metal is rougher than clean metal, you want to know its effective...
-
6 (a) Below is a diagram of a rotating disc viscometer (FIGURE 4). Explain its operations and limitations as to use. If, in a similar works situation, it is necessary to make measurements on a...
-
As part of your role in the Business Analytics and Data Analytics team, you have been asked to forecast Food Retailing as part of a wider report being commissioned by the above collaboration - on...
-
You are three students who have together bought a business that makes snow. The customers consist of both large public enterprises and private individuals. The business is run all year round, but the...
-
Question 4 25 p J Mart is considering purchasing a new inventory control system featuring state-of-the-art technology. Two vendors have submitted proposals to supply J Mart with the new system. The...
-
ME 2352 Design Optimization Assignment TWO, due February 6th, 2024, 4:00 pm University of New Brunswick Department of Mechanical Engineering 1. By use of definition of linear dependency determine if:...
-
A total of n balls are to be put into k boxes with the conditions that there will be n1 balls in box I, n2 balls in box 2, and so on, with nk balls being placed in box k (n1 + ... + nk = n) Explain...
-
In each of the following independent cases, document the system using whatever technique(s) your instructor specifies. a. Dreambox Creations (www.dreamboxcreations.com/) in Diamond Bar, California,...
-
The Lyman series in the hydrogen atom corresponds to transitions that originate from the n = 1 level in absorption or that terminate in the n = 1 level for emission. Calculate the energy, frequency...
-
Calculate the wavelengths of the first three lines of the Lyman, Balmer, and Paschen series, and the series limit (the shortest wavelength) for each series.
-
How many ways are there to place three electrons into an f sub-shell? What is the ground-state term for the f 3 configuration, and how many states are associated with this term? See Problem P 22.36.
-
Exercise 4-50 Theory of Constraints (LO 4-5) CompDesk, Inc., makes a single model of an ergonomic desk (with chair) for computer usage. The desk is manufactured in building 1, and the chair is...
-
In a capital account, explain which side (debit or credit) will decrease and which side will increase. Provide an example (just make one up) of a transaction with a T-account for each side of the...
-
How much sales revenue must ABC Catering generate in order to break

Study smarter with the SolutionInn App


