For the reaction BaCO 3 (s) BaO(s) + CO 2 (g), r G = +219.7
Question:
For the reaction BaCO3(s) → BaO(s) + CO2(g),
ΔrG° = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of Δf G° for BaCO3(s).
Data given in Appendix L
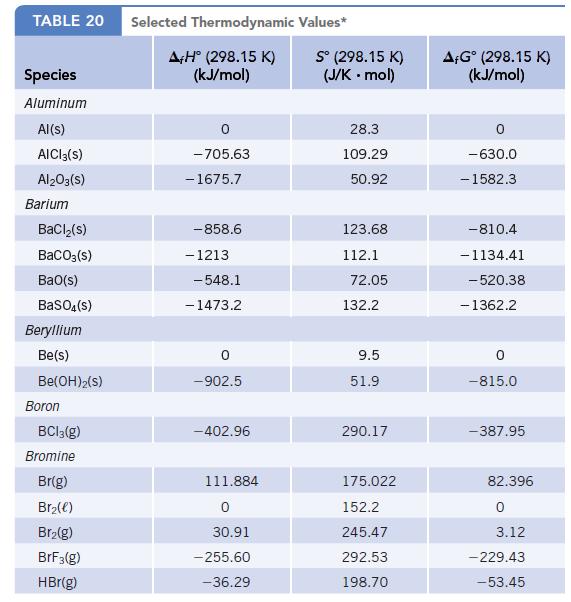
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+H° (298.15 K) (kJ/mol) -705.63 - 1675.7 -858.6 - 1213 -548.1 -1473.2 0 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K.mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 AFGᵒ (298.15 K) (kJ/mol) -630.0 - 1582.3 -810.4 -1134.41 -520.38 - 1362.2 0 -815.0 -387.95 82.396 0 3.12 -229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
BaCO3s BaOs CO2g AH for BaCO30 1213 KJmol 30 AH for Baos 5481 KJmol AH for CO2g 39...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For the reaction TiCl 2 (s) + Cl 2 (g) TiCl 4 (), r G = 272.8 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of f G for TiCl 2 (s). Data given in Appendix...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
DS Unlimited has the following transactions during August. August 6 Purchases 88 handheld game devices on account from GameGirl, Inc., for $290 each, terms 1/10, n/60. August 7 Pays $490 to Sure...
-
What advice would you give Michelle about the ethical issues involved in her project? Michelle had been a student union welfare officer on a years sabbatical from her undergraduate accounting and...
-
What is meant by customer requirements? Why must they be precise?
-
p. 510 Organizational restructuring efforts have a weak negative effect on job performance. They have a more significant negative effect on organizational commitment because employees tend to feel...
-
Whirlpool Corporation had the following abbreviated income statement for a recent year: (in millions) Net sales ..................$19,408 Cost of goods sold .............. 16,517 Selling...
-
Homework Brief Exercise 6-21 (Algo) Estimating stand-alone selling prices: adjusted market assessment approach [LO6-6) O'Hara Associates sells golf clubs, and with each sale of a ful set of clubs...
-
Determine whether the reactions listed below are entropy-favored or disfavored under standard conditions. Predict how an increase in temperature will affect the value of r G. (a) N 2 (g) + 2 O 2 (g)...
-
Using values of f H and S, calculate the standard molar free energy of formation, f G, for each of the following: (a) Ca(OH) 2 (s) (b) Cl(g) (c) Na 2 CO 3 (s) Compare your calculated values of f G...
-
Rework Problem 29 for an outside temperature of 20 o C
-
IKEA's People and Planet Positive sustainability plan, launched in 2012, aims to contribute to a better life for people and a better future for the planet. The plan outlines several sustainable goals...
-
Question 4 [25 marks] A cantilever beam AB is fixed to a wall and is subjected to concentrated and distributed loads as shown in figure B1. a) Draw the free-body diagram of the problem. [5 marks] a)...
-
GMC is an Australian farm machinery manufacturer, operating since 1975. The company makes high-quality farm machinery and equipment including a range of slashers, mowers, aerators, mulchers and...
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Repeat Problem 1.7.12 with the condition that one of the six people, Andrea, must sit next to Scott. In how many ways can the seating arrangements be made if Andrea refuses to sit next to Scott?...
-
How does Kant answer Humes bundle theory of self? Do you think he is successful?
-
Atomic emission experiments of a mixture show a calcium line at 422.673 nm corresponding to a 1 P 1 1 S 0 transition and a doublet due to potassium 2 P 3/2 2 S 1/2 and 2 P 1/2 2 S 1/2 transitions...
-
Consider the 1s np 3 P 1s nd 3 D transition in He. Draw an energy-level diagram, taking the spin-orbit coupling that splits terms into levels into account. Into how many levels does each term split?...
-
Calculate the transition dipole moment, for a transition from the 1s level to the 2p z level in H. Show that this transition is allowed. The integration is over r, θ, and . Use for the...
-
The duties or restrictions stated in Circular 230, Subpart B, do NOT include __________. Advertising that taxpayers may use pay stubs in lieu of Form W-2. Charging exorbitant fees. Knowingly...
-
Flexible Budgeting and Variance Analysis I Love My Chocolate Company makes dark chocolate and light chocolate. Both products require cocoa and sugar. The following planning information has been made...
-
Campbell pays a consultant $85,000 to assess whether it should open a new plant that would sell unique soups from around the world and that would invite visitors and make it a tourist destination....

Study smarter with the SolutionInn App


