Four balloons are each filled with a different gas, each having a different density: If the density
Question:
Four balloons are each filled with a different gas, each having a different density:
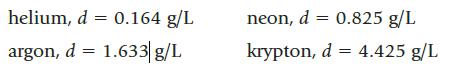
If the density of dry air is 1.12 g/L, which balloon or balloons float in air?
Transcribed Image Text:
helium, d = 0.164 g/L argon, d = 1.633 g/L neon, d = 0.825 g/L krypton, d = 4.425 g/L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
To determine which balloons float in air we need to compare the density of each gas...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balloons are often filled with helium gas because it weighs only about one-seventh of what air weighs under identical conditions. The buoyancy force, which can be expressed as Fb =...
-
A room is filled with saturated moist air at 25oC and a total pressure of 100 kPa. If the mass of dry air in the room is 100 kg, the mass of water vapor is (a) 0.52 kg (b) 1.97 kg (c) 2.96 kg (d)...
-
A room is filled with saturated moist air at 25C and a total pressure of 100 kPa. If the mass of dry air in the room is 100 kg, the mass of water vapor is (a) 0.52 kg (b) 1.97 kg (c) 2.96 kg (d) 2.04...
-
Repeat Example 13.1, but for electrophilic substitution at C-2 or C-4 of pyridine. Explain why substitution at C-3 (eq. 13.2) is preferred. 4 H
-
Using the data in Exercise 8-13, assume that during the second year of operations O'Hara Automotive Supply Co. had net sales of $4,800,000, wrote off $114,800 of accounts as uncollectible using the...
-
QUESTION 3 Ali, Ben and Cathy have been in partnership for many years sharing profits and losses based on the ratio 2:2:1. They provided the following information. Statement of Financial Position As...
-
6. Let [a, b] be a closed, bounded, nondegenerate interval. Find all functions I that satisfy the following conditions for some fixed O! > 0: I is continuous and I-Ion [a, b], I'(x) =I- 0 and I'(x) =...
-
At the time of his death this year on September 4, Kenneth owned the following assets. Fair Market Value City of Boston bonds $2,500,000 Stock in Brown Corporation 900,000 Promissory note issued by...
-
questions based on 10-k forms for the fiscal year ended january 30,2021. companies are amazon and target Determine the average days to collect accounts receivable for Year 3. Do you feel this is...
-
The photo below shows elemental iodine dissolving in ethanol to give a solution. Is this a physical or chemical change? Cengage Learning/Charles D. Winters Elemental iodine dissolving in ethanol.
-
Discuss the evidentiary problems raised by the following questions: a. After drinking tea made from tarragon leaves every day for a year, your grandfather reports that his bunions cleared up. Does...
-
A problems _____________________ will answer the question What does the user want to see displayed on the screen, printed on the printer, or stored in a file? a. Input b. Output c. Processing d....
-
Data: Sodium Systolic98 14799 14996 175109 14591 135107 14987 121110 170102 163103 141117 14992 13590 12793 132113 18199 152114 164103 14496 148111 180128 18392 13284 135102 141103 147117 16789...
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
List all paths and calculate and determine the critical path for the Gathering Information PERT diagram.
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
Provide an IUPAC name for each of the following alcohols: a. b. c. d. e. HO. Br Br
-
Mandelate esters exhibit spasmolytic activity (they act as muscle relaxants). The nature of the alkyl group (R) greatly affects potency. Research indicates that the optimal potency is achieved when R...
-
Draw the alkoxide formed in each of the following cases: a. b. c. d. HO ? Na NaH
-
When analyzing the results of substantive procedures, auditors should beware of: O professional skepticism. confirmation bias. audit engagement deadlines. weak internal controls
-
what is are the answers to a, b and c for 23-32P?
-
On December 31, 2016 Apple company decides that an allowance for doubtful accounts is required in the amount of $6,000. There is zero balance in the Allowance for Doubtful Account. What would be the...

Study smarter with the SolutionInn App


