Draw the alkoxide formed in each of the following cases: a. b. c. d. HO ? Na
Question:
a.
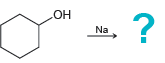
b.
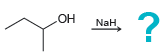
c.
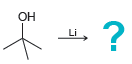
d.
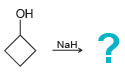
Transcribed Image Text:
HO ? Na NaH ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
a b...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a diastereomer for each of the following a. b. c. d. CH H-OH 8 CH3CH2 CH3 H3C CH3 -
-
Draw the keto tautomer for each of the following: a. b. c. d. CH3CH-CCH3 CH3CH2CH2C-CH -CHOH
-
The first step in eq. 10.26 really involves two reactions, addition of ammonia to the carbonyl carbon to form an ammonium alkoxide followed by a proton transfer from the nitrogen to the alkoxide...
-
John Deer began a restaraunt consulting company. Below are events and transactions that occurred during the first month of operation. May 1 John Deer invested $38,000 cash to start a consulting...
-
Why did the FDIC adopt Prompt Corrective Action procedures in the 1990s?
-
A company can advertise through radio, TV, or newspaper. The weekly costs of advertising on the three media are estimated at $200, $900, and $300, respectively. The company can classify its sales...
-
What is the difference between a discrete probability distribution and a continuous probability distribution? AppendixLO1
-
Olthof Company purchased 15,000 pounds of raw materials at $3.00 per pound. The companys standard cost per pound is $2.50. Calculate the materials price variance for this purchase.
-
Lee Company has the following information for the pay period of December 1531: Gross payroll $18,703 Federal income tax withheld $2,993 Social security rate 6% Federal unemployment tax rate 0.8%...
-
Do you think that information appliances like PDAs will replace personal computers (PCs) in business applications? Explain.
-
Mandelate esters exhibit spasmolytic activity (they act as muscle relaxants). The nature of the alkyl group (R) greatly affects potency. Research indicates that the optimal potency is achieved when R...
-
Use the aggregate supply-aggregate demand model to determine which of the following will lead to higher aggregate output. a. A tax increase b. A spike in world oil prices c. A cut in interest rates...
-
A tunnel is modeled as an air-filled metallic rectangular waveguide with dimensions a = 8 m and b = 16 m. Determine whether the tunnel will pass: (a) A 1.5-MHz AM broadcast signal, (b) A 120-MHz FM...
-
What is the discount rate? PV = 7 0 0 ; t = 5 year period; FV = 1 0 0 0
-
How is planning illustrated in this case story? How is strategic management illustrated in this case story? The new CEO stated that the CEO's job is to give employees a point of view. Explain what...
-
Explain the Following Questions: 1. What essential characteristics exist in a proper understanding of "personal mastery," so that as an individual achieves greater progress in this discipline, they...
-
Few people want to eat discolored french fries. Potatoes are kept refrigerated before being cut for french fries to prevent spoiling and preserve flavor. But immediate processing of cold potatoes...
-
Part 3 of 4 Points: 0.49 of 1 Compute P(X) using the binomial probability formula. Then determine whether the normal distribution can be used to estimate this probability. If so, approximate P(X)...
-
Verify that f(x) = 2x - 1 and are inverses. 17 x = (x) -f +
-
A simple random sample of 220 university students were asked what pasta they usually order and with which sauce. The preferences of these respondents are summarised below: Sauce Bolognese Pasta...
-
How could you prepare benzyl phenyl ether from benzene and phenol? More than one step is required.
-
When 2-methyl-2, 5-pentanediol is treated with sulfuric acid, dehydration occurs and 2, 2-dimethyltetrahydrofuran is formed. Suggest a mechanism for this reaction. Which of the two oxygen atoms is...
-
Write the mechanism of the hydrolysis of cis-5, 6-epoxydecane by reaction with aqueous acid. What is the stereochemistry of the product, assuming normal backside SN2 attack?
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


