Give the systematic name for the following alkane. Draw a structural isomer of the compound, and give
Question:
Give the systematic name for the following alkane. Draw a structural isomer of the compound, and give its name.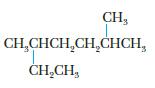
Transcribed Image Text:
CH₂ CH₂CHCH₂CH₂CHCH, CH₂CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
H H H CH3 ...View the full answer

Answered By

Rajesh Kumar Debariki
Bachelor of arts student and an a freelancer of Chegg. Bartleby etc.
Highlight Impressive Experience and Accomplishments.
I am a self-starter with strong interpersonal skills. I work efficiently both as an individual contributor as well as along with a team. I seek new challenges and try to think out-of-the-box while looking for creative solutions to a given problem...
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Give the systematic name for each of the following compound a. CH3C==CCH2CH2CH2CH==CH2 b. c. CH3CH2C==CCH2CH2C==CH d. e. HOCH CH CH2CH C-C CI CH3
-
Elaborate above statement The more specific you are about the feedback you need, the more helpful the feedback is likely to be.
-
Draw all the isomers that have the molecular formula C5H11Br(Hint: There are eight such isomers.) a. Give the systematic name for each of the isomers. b. Give a common name for each isomer that has...
-
On January 5, Jones Ventures Inc. purchased 40% of the outstanding stock of Pilots Manufacturing Corp. The purchase was 20,000 shares at $10 per share. Jones received dividends from Pilots in the...
-
The net income reported on the income statement of Ground Hog Co. was $1,250,000. There were 250,000 shares of $40 par common stock and 50,000 shares of $10 preferred stock outstanding throughout the...
-
Tim Angel opened a small travel agency. At the end of its second year of operation, Angel Travel, Inc., had the trial balance shown below. The following information is also available: a. Office...
-
Insert the missing words: In future, new recruits will arrive at an organization with the key question . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . and feel quite comfortable...
-
Bottom Dollar Furniture, Inc. completed the following treasury stock transactions in 2016: Dec. 1 Purchased 1,800 shares of the company's $1 par value common stock as treasury stock, paying cash of...
-
On January 1, 2021, Tara, Lauren, and Christen form a business, Hawks, as a general partnership. They contribute the following to the partnership: Tara contributes land with a fair market value of...
-
Draw the structure of each of the following compounds: (a) 2,3-dimethylhexane (b) 2,3-dimethyloctane (c) 3-ethylheptane (d) 3-ethyl-2-methylhexane
-
Isooctane, 2,2,4-trimethylpentane, is one of the possible structural isomers with the formula C 8 H 18 . Draw the structure of this isomer, and draw and name structures of two other isomers of C 8 H...
-
Briefly describe the three types of processor scheduling.
-
Sometimes when we are asked for a linear model, the information that we are given is data about a scenario. In these cases we have to use Excel to generate a trendline. There is a video in this...
-
1. Purpose Explain 3 points from the Introduction section as to why this study is important. How did this study build on the existing literature in this area? 2. Participants Outline at least 2...
-
In this Capstone experience, you will develop a strategy playbook for a selected organization. You may be familiar with the concept of a playbook as it relates to a sports team, but what might that...
-
On January 1, 2024, the general ledger of Big Blast Fireworks includes the following account balances: Accounts Cash Debit Credit $25,900 Accounts Receivable 46,500 Allowance for Uncollectible...
-
The WRX can travel 1 / 4 of a mile in 1 3 . 9 sec . Calculate the acceleration over this distance if assumed constant.
-
Derive the formula l2 + 22 + 32 + ... + n2 = n(n + 1) (2n +1)/6 Using the following steps. (a) Show that (k + l)3 - k3 = 3k2 + 3k + 1. (b) Show that [23 - 13] + [33 - 23] + [43 - 33] + ... + [(n +...
-
Organizations are increasing their use of personality tests to screen job applicants. What are some of the advantages and disadvantages of this approach? What can managers do to avoid some of the...
-
When a fighter pilot makes a very quick turn, he experiences a centripetal acceleration. When this acceleration is greater than about 8 g, the pilot will usually lose consciousness (black out)....
-
When a planet orbits around a star, the star also moves in an orbit. Since it is much more massive than the planet, the stars orbital radius r star is much smaller than that of the planet r planet ....
-
At a practice for a recent automobile race, officials found that the drivers were nearly blacking out, which led to cancellation of the race. The cars were traveling at about 240 mi/h, and the track...
-
Current Attempt in Progress Craig Company asks you to review its December 3 1 , 2 0 2 0 , inventory values and prepare the necessary adjustments to the books. The following information is given to...
-
Define the two types of hot assets that a partnership might own.
-
Complete the table for the year ended December 31,2022 . The company depreciates all assets using the half-year conventio answers to 0 decimal places, e.g. 45,892. Kingbird Industries presents you...

Study smarter with the SolutionInn App


