The density of pure water at various temperatures is given below Suppose your laboratory partner tells you
Question:
The density of pure water at various temperatures is given below 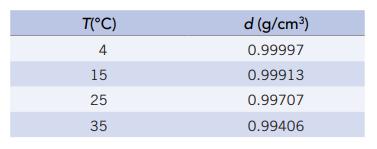
Suppose your laboratory partner tells you the density of water at 20°C is 0.99910 g/cm3. Is this a reasonable number? Why or why not?
Transcribed Image Text:
T(°C) 4 15 25 35 d (g/cm³) 0.99997 0.99913 0.99707 0.99406
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To determine whether the reported density of water at 20C 099910 gcm is reaso...View the full answer

Answered By

Salmon ouma
I am a graduate of Maseno University, I graduated with a second class honors upper division in Business administration. I have assisted many students with their academic work during my years of tutoring. That has helped me build my experience as an academic writer. I am happy to tell you that many students have benefited from my work as a writer since my work is perfect, precise, and always submitted in due time. I am able to work under very minimal or no supervision at all and be able to beat deadlines.
I have high knowledge of essay writing skills. I am also well conversant with formatting styles such as Harvard, APA, MLA, and Chicago. All that combined with my knowledge in methods of data analysis such as regression analysis, hypothesis analysis, inductive approach, and deductive approach have enabled me to assist several college and university students across the world with their academic work such as essays, thesis writing, term paper, research project, and dissertation. I have managed to help students get their work done in good time due to my dedication to writing.
5.00+
4+ Reviews
16+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A look at the harmful effects of too much fast food is an interesting question as to whether the fastfood restaurants should be held liable for those health problems Do you agree or disagree Why or...
-
The vapor pressure of water at various temperatures is in Table E11-11: (a) Draw a scatter diagram of these data. What type of relationship seems appropriate in relating y to x? (b) Fit a simple...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
You have just completed your four-year degree at LLC University! Your student loans that you have accumulated while studying at LLC total $25,000. Since you have graduated, you must now begin...
-
1. What are the ethical issues involved in pizza deliveries to dangerous neighborhoods that are often predominantly inhabited by minorities? What tensions exist between economic and ethical issues?...
-
Henrich is a single taxpayer. In 2021, his taxable income is $454,500. What is his income tax and net investment income tax liability in each of the following alternative scenarios? Use Tax Rate...
-
5. [RECIPROCAL RULE] Suppose that f is differentiable at a and f (a) # O. (a) Show that for h sufficiently small, f(a + h) # O. (b) Using Definition 4.1 directly, prove that 1/ f(x) is differentiable...
-
An analysis of the payroll for the month of November for CinMar Inc. reveals the information shown: Andrews, Lomax, and Herzog are production workers, and Dimmick is the plant manager. Hendrick is in...
-
Connor Products manufactures three types of remote-control devices: Eronomy, Standart, and Delane. The company, whom related cost drivers). Each activity, its budgeted cost, and related cont driver...
-
When you heat popcorn, it pops because it loses water explosively. Assume a kernel of corn, with a mass of 0.125 g, has a mass of only 0.106 g after popping. (a) What percentage of its mass did the...
-
Diamond has a density of 3.513 g/cm 3 . The mass of diamonds is often measured in carats, where 1 carat equals 0.200 g. What is the volume (in cubic centimeters) of a 1.50-carat diamond?
-
Olympus Equipment Company purchased a new piece of factory equipment on May 1, 2011, for $29,200. For income tax purposes, the equipment is classified as a 7-year asset. Because this is similar to...
-
3. (3pt.) A state of a physical system is just a description of the system at an instant in time in terms of its properties. In classical mechanics, states are represented by points (in phase space)....
-
1. The KYM company wants to invest $ 523,000 pesos in the bank that guarantees a simple interest rate of 3.32% quarterly. If the company is considering the 8-month investment. What amount will you...
-
3. (5 points) The uncertainty principle limits our ability to determine simultaneously the position and momentum of a particle. (a) Why were classical physicists unaware of the limitations that this...
-
CASE STUDY Patient Name Valarie Ramirez Attending Paul F. Buckwalter, MD PATIENT INFORMATION DOB 08/04/1986 Allergies MAN 00-AA-006 Penicillin Other Information Past HX: AB x1 Valarie Ramirez arrives...
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: SalesProduction CostOperating Expenses 20191 st Quarter P280,000P192,000P64,000 2 nd Quarter 320,000...
-
What are the advantages of using mind mapping to facilitate project planning?
-
Consider a game of poker being played with a standard 52-card deck (four suits, each of which has 13 different denominations of cards). At a certain point in the game, six cards have been exposed. Of...
-
In Section 4.2, we learned how to name bicyclic compounds. Using those rules, together with the rules discussed in this section, provide a systematic name for the following bicyclic compound:
-
Classify each of the following alkenes as mono-substituted, di-substituted, tri-substituted, or tetra-substituted: a. b. c. d. e.
-
Three of the compounds from Problem 13.14 can be prepared from the reaction between a hydride reducing agent (NaBH 4 or LAH) and a ketone or aldehyde. Identify those three compounds, and explain why...
-
Schoeck, CPA, is considering leaving a position at a major public accounting firm to join the staff of local bookkeeping and advisory firm that does write-up work, tax preparation and planning, and...
-
board Which entry requires three or more accounts? O a. simple entry. 30 O b. multiple entry. O c. triple entry. O d. compound entry
-
During a sales call, Tune Products, Inc., offers to sell to Unlimited Sales Company one hundred MP3 players at $50 a piece, subject to certain specific delivery dates. Unlimited replies with a signed...

Study smarter with the SolutionInn App


