The enthalpy changes for the following reactions can be measured: (a) Use these values and Hesss law
Question:
The enthalpy changes for the following reactions can be measured: 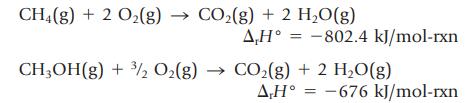
(a) Use these values and Hess’s law to determine the enthalpy change for the reaction![]()
(b) Draw an energy level diagram that shows the relationship between the energy quantities involved in this problem.
Transcribed Image Text:
CH4(g) + 2 O₂(g) → CO₂(g) + 2 H₂O(g) A,H° = -802.4 kJ/mol-rxn CH₂OH(g) + 3/2 O₂(g) → CO₂(g) + 2 H₂O(g) AH° -676 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Energy a AH 126 kJmolrxn b CH4g 12 O...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy changes of the following reactions can be measured: (a) Use these values and Hesss law to determine the enthalpy change for the reaction (b) Draw an energy level diagram that shows the...
-
The standard molar enthalpy of formation of diborane, B 2 H 6 (g), cannot be determined directly because the compound cannot be prepared by the reaction of boron and hydrogen. It can be calculated...
-
The standard enthalpy of formation of solid barium oxide, BaO, is 553.5 kJ/mol, and the standard enthalpy of formation of barium peroxide, BaO 2 , is 634.3 kJ/mol. (a) Calculate the standard enthalpy...
-
Please help me calculate the current assets and current liabilities. Cash and cash equivalents Deposits Marketable securities Inventory Property & equipment, net Loan to shareholders Notes receivable...
-
Your company has received an order for 20 units of a product. The labor cost to produce the item is $9.50 per hour. The setup cost for the item is $60 and material costs are $25 per unit. The item is...
-
As seen in Problem 3.109, silicon carbide nanowires of diameter D = 15 nm can be grown onto a solid silicon carbide surface by carefully depositing droplets of catalyst liquid onto a flat silicon...
-
Samples of size 5 provided the following 20 sample means for a production process that is believed to be in control. 95.72 95.24 95.18 95.44 95.46 95.32 95.40 95.44 95.08 95.50 95.80 95.22 95.56...
-
1. How would you explain the abrupt change in Elisas customer service behaviour? Outline what has happened to her motivation using expectancy and equity theories. 2. Could Elisas employer have...
-
prepare a journal entry for the following transaction narrative: On July 31st, 2020 La Nouveau paid the first quarterly interest on bonds.
-
A 0.692-g sample of glucose, C 6 H 12 O 6 , was burned in a constant-volume calorimeter. The temperature rose from 21.70C to 25.22C. The calorimeter contained 575 g of water, and the bomb had a heat...
-
Suppose you burned 1.500 g of benzoic acid, C 6 H 5 CO 2 H, in a constant-volume calorimeter and found that the temperature increased from 22.50C to 31.69C. The calorimeter contained 775 g of water,...
-
The following problems provide more practice on operations with fractions and decimals. Perform the indicated operations. 3 4 -6
-
PP Company purchases a material that is then processed to yield three chemicals: anarol, estyl, and betryl.In June, PPC purchased 10,000 gallons of the material at a cost of $250,000, and the company...
-
Suppose Boyson Inc. free cash flow for the next year is $ 1 5 0 , 0 0 0 and the FCF is expected to grow a concert rate of 6 . 5 % if WACC is 1 2 . 5 % what is the market value of the firm?
-
An eight lane urban freeway (four lanes in each direction) is on rolling terrain and has 11-ft lanes with a 4-ft right-side shoulder. The interchange density is 1.25 per mile. The base free-flow...
-
For the following business events, please indicate the increase (+) or decrease (-) on the following income statement and balance sheet categories. If there is no effect, leave the box blank. If...
-
4. Change the magnet to the original orientation and drag through the coil. a. What happens to the voltage and light bulb as the North Pole moves through the coil? b. What happens to the voltage and...
-
Answer the following questions on internal control: a. Separation of duties is an important internal control procedure. Why is this so? b. Cash may be a small item on the financial statements....
-
Bobbie Singh provides writing services for small businesses. He blogs for companies that need professionally written content. His business records at November 15, 2023, are shown below: During the...
-
The molar heat capacity C P,m of SO 2 (g) is described by the following equation over the range 300 K < T < 1700 K: In this equation, T is the absolute temperature in kelvin. The ratios T/K ensure...
-
Use the relation (U/V ) T = T(P/T) V P and the cyclic rule to obtain an expression for the internal pressure, (0U/0V )T , in terms of P, , T, and .
-
A mass of 34.05 g of H 2 O(s) at 273 K is dropped into 185 g of H 2 O(l) at 310.K in an insulated container at 1 bar of pressure. Calculate the temperature of the system once equilibrium has been...
-
Problem 2-9 A Preparing financial statements from a trial balance 106 Credit Hipster Optical Trial Balance May 31, 2020 Account Title Cash Accounts receivable office supplies Prepaid insurance office...
-
what's the answer ABC Corp factors $586,905 of accounts receivable with a financing company on a 'with recourse' basis. The financing company will collect the receivables. The receivable records are...
-
Problems Group A P-F:1-41A. Using the accounting equation for transaction analysis (Learning Objective 4) Meg McKinney opened a public relations firm called Solid Gold on August 1, 2024. The...

Study smarter with the SolutionInn App


