The mass spectrum of phosphoryl chloride, POF 3 , is illustrated here. (a). Identify the cation fragment
Question:
The mass spectrum of phosphoryl chloride, POF3, is illustrated here.
(a). Identify the cation fragment at a m/Z ratio of 85.
(b). Identify the cation fragment at a m/Z ratio of 69.
(c). Which two peaks in the mass spectrum provide evidence that the oxygen atom is connected to the phosphorus atom and is not connected to any of the three fluorine atoms?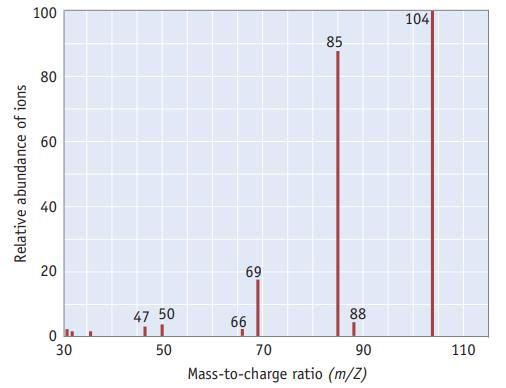
Transcribed Image Text:
Relative abundance of ions 100 80 60 40 20 0 30 47 50 50 69 66 70 85 88 90 Mass-to-charge ratio (m/Z) 104 110
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Identify the cation fragment at a mZ ratio of 85 The cation fragment at a mZ ratio of 85 is POF2 T...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The mass spectrum of unknown compound A shows a molecular ion at m/z 116 and prominent peaks at m/z 87 and m/z 101. Its UV spectrum shows no maximum above 200 nm. The IR and NMR spectra of A follow....
-
In the mass spectrum of 2, 6-dimethyl-4-heptanol there are prominent peaks at m/z 87, 111, and 126. Propose reasonable structures for these fragment ions.
-
The following spectra for A and B correspond to two structural isomers. The NMR singlet at (1.16 in spectrum A disappears when the sample is shaken with D2O.The singlet at (0.6 ppm in the spectrum of...
-
Why is it helpful to understand leadership as a theory while managing a healthcare-orientated organization? Justify your stance using two examples. What factors do you think should appear in a model...
-
What is the purpose of an operational environment and a test environment?
-
nWhat are these companies' SCAS? [Homework #2.1] Samsung, Apple, Alibaba, Tesla nWhich sources of SCA are growing in importance during 2021-2022 periods? [Homework #2.2] Any example
-
Explore the scope of HR planning? LO1
-
Consider the following data for a project never before attempted by your company: a. Draw the network diagram for this project. b. Identify the critical path and estimate the projects duration. c....
-
Bathworks Budgeted Income Statement For the Year Ended December 31 $ 102,000 $ 1,600 68,800 $ 70,400 2,400 Sales Cost of goods sold: Finished goods inventory, beginning * Cost of goods manufactured...
-
You combine 1.25 g of germanium, Ge, with excess chlorine, Cl 2 . The mass of product, Ge x Cl y , is 3.69 g. What is the formula of the product, Ge x Cl y ?
-
The mass spectrum of nitrogen dioxide is illustrated here. (a) Identify the cations present for each of the four peaks in the mass spectrum. (b) Does the mass spectrum provide evidence that the two...
-
Arantxa Corporation has outstanding 20,000 shares of R\($5\) par value ordinary shares. On August 1, 2015, Arantxa reacquired 200 shares at R\($80\) per share. On November 1, Arantxa reissued the 200...
-
Economic order quantity; order cost; carrying cost Starr Company predicts that it will use 360,000 units of material during the year. The material is expected to cost $5 per unit. Starr anticipates...
-
Requirement 1. Prepare a horizontal analysis of the comparative income statement of McCormick Designs, Inc. Round percentage changes to one decimal place. (Round the percentages to one decimal place,...
-
GAAP looks to compare entities with an apples-to-apples valuation so that the entities can be viewed by interested parties to compare financial values and how effectively and efficiently they use...
-
Prepare a statement of cash flows for Wu using the indirect method.
-
A plate of steel with a central through-thickness flaw of length 16 mm is subjected to a stress of 350 MPa normal to the crack plane. If the yield strength of the material is 1400 MPa what is the...
-
On January 1, 2014, Paxton Company purchased a 70% interest in Sagon Company for $1,300,000, at which time Sagon Company had retained earnings of $500,000 and capital stock of $1,000,000. On January...
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
For each of the following descriptions draw the structure of a compound that fits the description. (Note: There are many correct answers for each of these problems.) a) An alkyl halide that produces...
-
Assuming that H f is constant in the interval 275 K 600. K, calculate G for the process *H2O, g, 298 K) (H 2 O, g, 600, K). Calculate the relative change in the Gibbs energy.
-
How many different alkenes will be produced when each of the following substrates is treated with a strong base? a) 1-Chloropentane b) 2-Chloropentane c) 3-Chloropentane d) 2-Chloro-2-methylpentane...
-
Are the following required to file a Federal return? Answer ( YES or NO ) HOH, age 29, gross income $18,850. Choose one answer. a. Yes b. No
-
Consider how Cherry Valley, a popular ski resort, could use capital budgeting to decide whether the $ 9 million Waterfall Park Lodge expansion would be a good investment. ( Click the icon to view the...
-
Straight-line depreciation on equipment is an example of a mixed cost O fixed cost O variable cost O step cost

Study smarter with the SolutionInn App


