Use standard reduction potentials (Appendix M) for the half-reactions AgBr(s) + e Ag(s) + Br
Question:
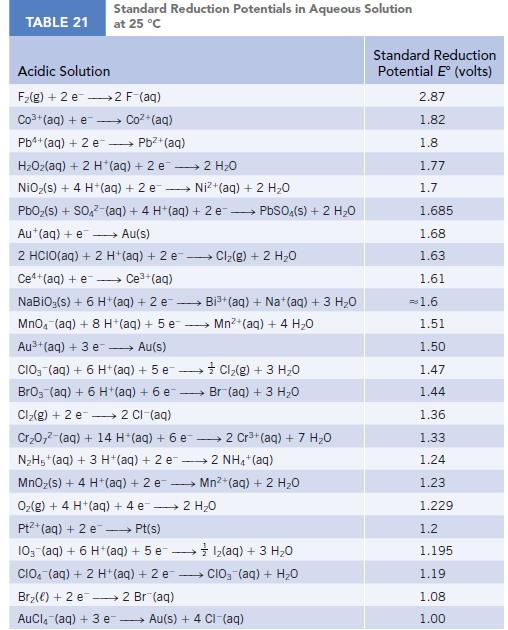
Use standard reduction potentials (Appendix M) for the half-reactions AgBr(s) + e− → Ag(s) + Br−(aq) and Ag+(aq) + e− → Ag(s) to calculate the value of Ksp for AgBr.
Data given in Appendix M
Transcribed Image Text:
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+ (aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+ (aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+ (aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO3(aq) + 6 H+ (aq) + 5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂ (s) + 4 H+ (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4H₂O Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
2303 RT E log Ksp nF nFE log Ksp 2303 RT 1x9...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
A rectangular pontoon 10m long 7m broad & 2.5m deep weights 686.7KN. It carries on its upper deck an empty boiler of 5m diameter weighing 588.6 KN. The centre of gravity of the boiler and pontoon are...
-
Koko Chocolate Company makes dark chocolate and light chocolate. Both products require cocoa and sugar. The following planning information has been made available: Koko Chocolate does not expect...
-
Choose a company with which you are familiar that manufactures a product. In this activity, you will be making reasonable assumptions about the activities involved in the value chain for this...
-
YourFire, Inc., is a small business owned by Curt and Julie Robards. Based in Brisbane, Australia, YourFire manufactures and sells a lightweight camping stove called the YourFire. Curt, who...
-
Staci Valek began dabbling in pottery several years ago as a hobby. Her work is quite creative, and it has been so popular with friends and others that she has decided to quit het job is $3,800 per...
-
2. The controller of Sensor Co. has also decided to implement an ABC system in the testing department She identifies the following three cost pools and cost drivers Costs Product A 33 Producto 15.000...
-
Diagram the apparatus used to electrolyze molten NaCl. Identify the anode and the cathode. Trace the movement of electrons through the external circuit and the movement of ions in the electrolysis...
-
Calculate r G and the equilibrium constant for the following reactions. (a) Zn+ (aq) + Ni(s) Zn(s) + Ni+ (aq) (b) Cu(s) + 2 Ag+ (aq) = Cu+ (aq) + 2 Ag(s)
-
A particular thermal system involves three objects of fixed shape with conduction resistances of R 1 = 1 K/W, R 2 = 2 K/W and R 3 = 4 K/W, respectively. An objective is to minimize the total thermal...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
A matrix A is said to have band width k if all entries that are more than k slots away from the main diagonal are zero: aij = 0 whenever |i - j| > k. (a) Show that a tridiagonal matrix has band...
-
Read the case study Richter: Information Technology at Hungarys Largest Pharma and answer the following question: How does the organization ensure the accuracy of the data it stores?
-
A hockey puck slides along a rough, icy surface. It has an initial velocity of 35 m/s and slides to a stop after traveling a distance of 95 m. Find the coefficient of kinetic friction between the...
-
A rock is dropped from a very tall tower. If it takes 4.5 s for the rock to reach the ground, what is the height of the tower?
-
A baseball is hit directly upward with an initial speed of 45 m/s. Find the velocity of the ball when it is at a height of 40 m. Is there one correct answer for v or two? Explain why.
-
8N please highligjt answer Profits have been decreasing for several years at Pegasus Airlines. In an effort to improve the company's performance, the company is thinking about dropping several...
-
On January 1, 20X1, Toy inc. Issued $500,000 of convertible bonds. The bonds mature on December 31, 20X5, Interest is payable annually at 6.0% on December 31. The bonds are convertible at the...
-
Exercise 18-11 (Algo) Balance sheet identification and preparation LO P1 End-of-year current assets for two different companies follow. One is a manufacturer, Rayzer Skis Manufacturing, and the...

Study smarter with the SolutionInn App


